The quest for sustainable energy solutions has catapulted hydrogen gas to the forefront of green technology discourse. Renowned for its high energy density and lack of carbon emissions when utilized, hydrogen embodies potential as a clean fuel alternative for the future. Indeed, hydrogen is the most plentiful element in the universe. However, its sheer abundance is not the entire narrative; hydrogen predominantly exists in bound forms, existing as part of compounds like ammonia and metal hydrides. This intrinsic nature triggers a pivotal question: how can we efficiently extract hydrogen from these compounds for widespread, practical use?
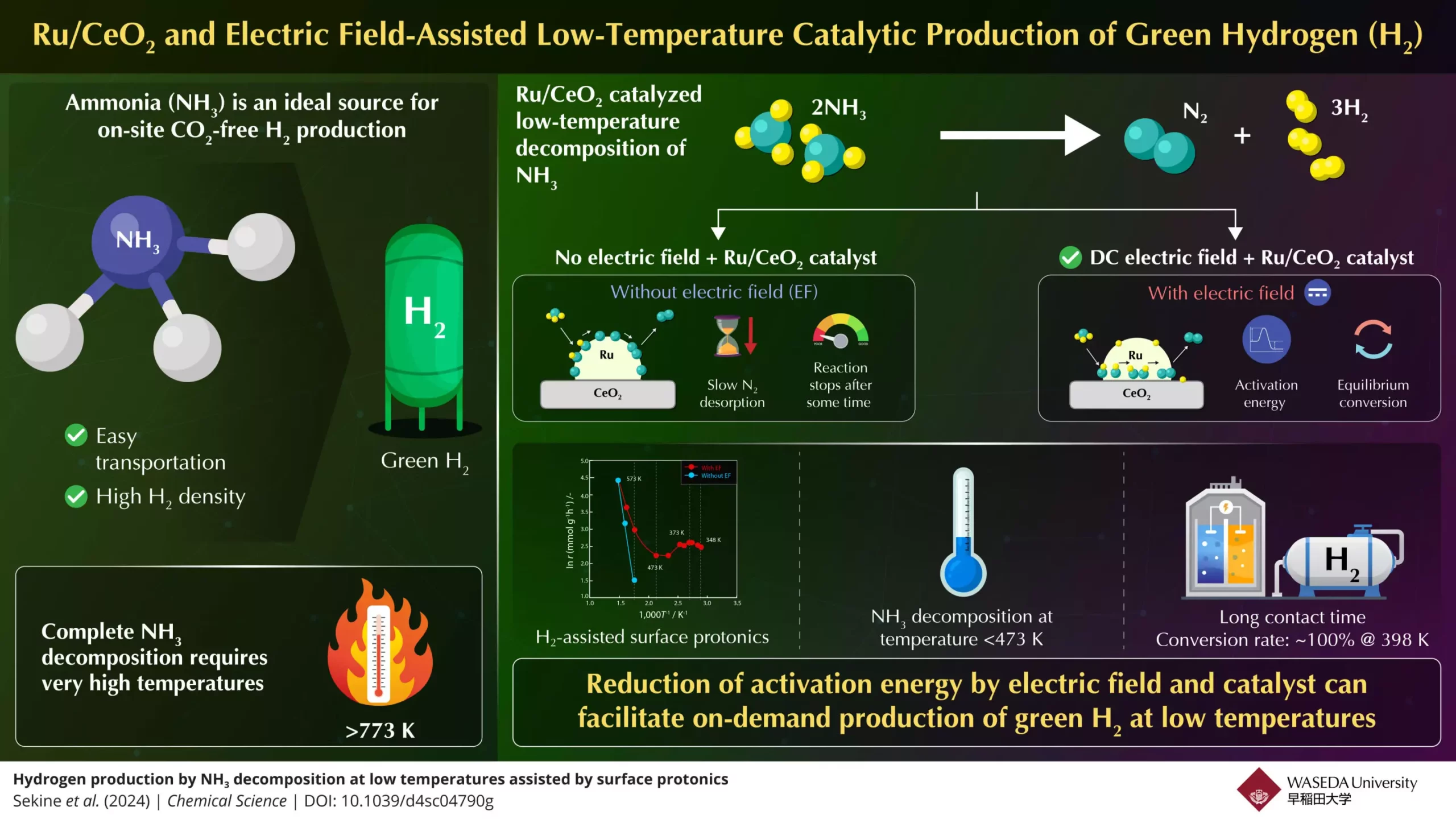
Among various hydrogen carriers, ammonia has emerged as a noteworthy candidate. It boasts significant advantages: ammonia contains about 17.6% hydrogen by weight and is relatively easy to liquefy and transport. These properties position ammonia as a potential hero in the green hydrogen sphere, however, a significant challenge persists. The conventional methods for decomposing ammonia to release hydrogen require exceedingly high temperatures—exceeding 773 K—which limits its practicality in real-world applications. Thus, the dream of utilizing ammonia for on-demand hydrogen generation remains partly unrealized.
Recent advancements in ammonia decomposition technology hint at a breakthrough in overcoming these hurdles. A collaborative research team from Waseda University and Yanmar Holdings, led by Professor Yasushi Sekine, has introduced a groundbreaking approach that operates at substantially lower temperatures. They presented an experimental framework capable of achieving efficient ammonia-to-hydrogen conversion by leveraging electric fields in tandem with a highly effective catalyst known as Ru/CeO2.
A transformative aspect of their method involves a detailed examination of the chemical reactions involved in ammonia decomposition. The team identified that nitrogen desorption becomes a bottleneck at low temperatures while the dissociation of nitrogen-hydrogen bonds occurs predominantly at higher temperatures. By integrating electric field assistance into their catalytic reactions, they advanced the proton conductivity on the catalyst’s surface, thus reducing the energy needed for these reactions to take place.
In their pioneering studies published in Chemical Science, the researchers demonstrated that their innovative design could facilitate the decomposition of ammonia below 473 K. Remarkably, with sufficient contact time between the ammonia feed and the catalyst, they achieved an outstanding 100% conversion rate at a mere 398 K—exceeding conventional equilibrium limitations. This efficiency stems from the electric field’s ability to enhance surface protonics, enabling proton hopping on the catalyst surface, significantly lowering the activation energy needed for ammonia conversion.
Conversely, the absence of an electric field hampered the nitrogen desorption process, leading to a stalling of the ammonia reaction over time. Their findings were supported by an extensive series of experimental tests and density functional theory calculations, underscoring the role of surface protonics as a critical component in the enhancement of ammonia’s conversion rates.
The implications of Sekine’s team’s research are profound, suggesting that ammonia can indeed serve as a reliable source for generating green hydrogen efficiently at lower temperatures. This innovative pathway promises to ease the hurdles associated with hydrogen production from ammonia, thereby facilitating a more seamless transition toward clean fuels. The potential for widespread adoption of CO2-free hydrogen could finally come within reach, marking a substantial step forward in combating climate change and fostering sustainable energy dependence.
Ultimately, this research not only highlights viable technologies for converting ammonia to hydrogen but also opens avenues for further exploration in energy systems. As we continue to grapple with the complexities of climate change and energy shortages, the development showcased by Sekine and his team proves to be an encouraging signal that effective solutions may soon become mainstream. The future of renewable energy, particularly through the lens of hydrogen production, is beginning to shine brightly on the horizon.

Leave a Reply