In a recent groundbreaking study published in Nature Communications by researchers from the Interface Science Department at the Fritz Haber Institute, a revolutionary advancement in the fight against climate change has been introduced. The study, titled “Reversible metal cluster formation on Nitrogen-doped carbon controlling electrocatalyst particle size with subnanometer accuracy,” presents a novel method for understanding the mechanisms of carbon dioxide (CO2) re-utilization for the production of fuels and chemicals. This research opens up new possibilities for the optimization of catalytic processes driven by renewable electricity.
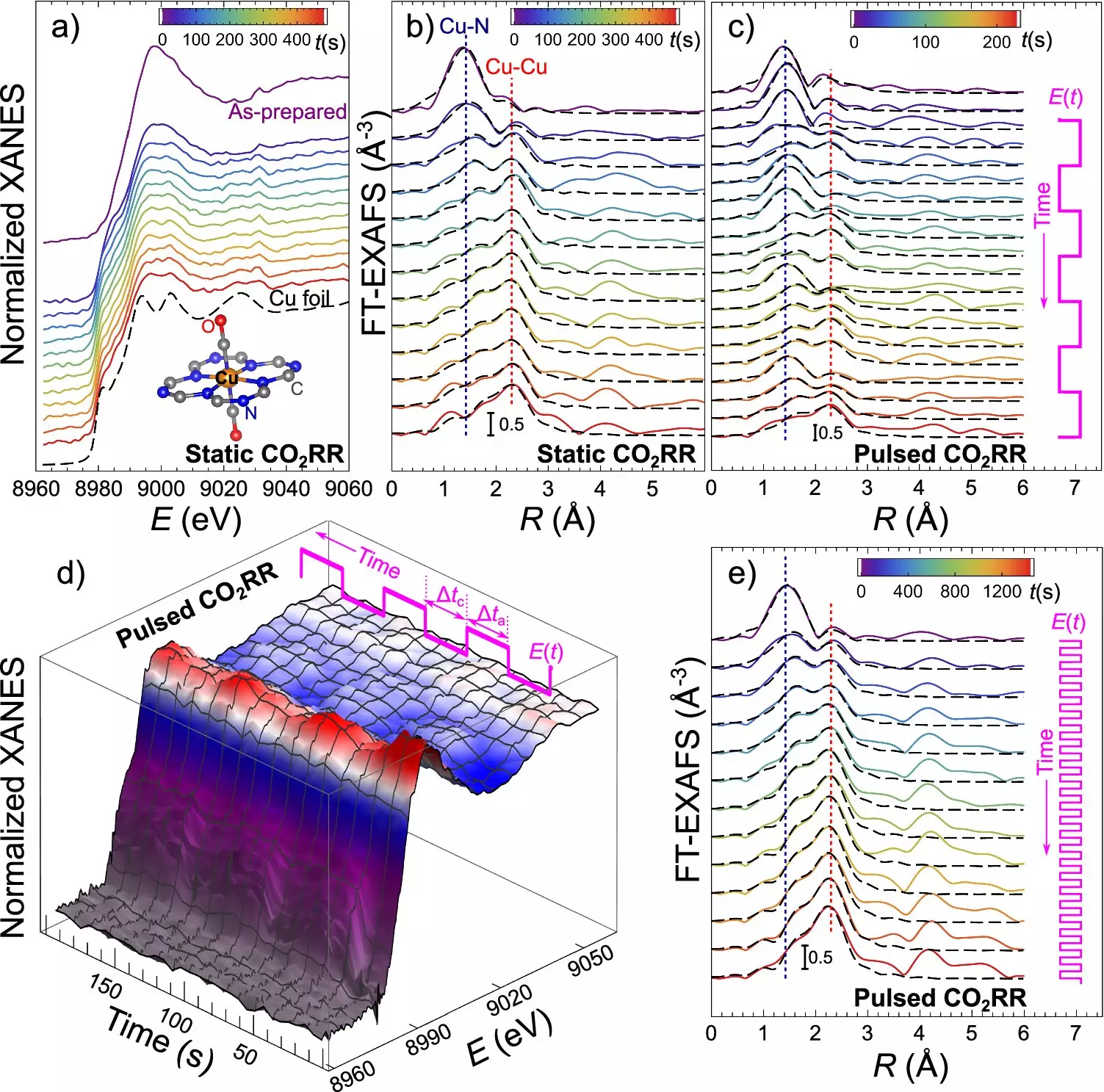
At the heart of this discovery is the unique properties of catalysts comprising ultradispersed copper and nitrogen atoms integrated into carbon matrices. During the electrocatalytic CO2 reduction (CO2RR) process, these catalysts display the ability to transition dynamically from having copper in the form of single atoms to the formation of small clusters and metal particles, commonly referred to as nanoparticles, and back again. This reversible transformation is triggered by changes in the electrical potential applied to the catalyst, offering unprecedented control over the catalyst’s structure and, consequently, the outcome of the CO2RR process.
One of the significant hurdles in scaling up CO2RR technology for practical applications has been the broad distribution of different reaction products, making it challenging to produce specific industrially relevant chemicals and fuels efficiently. However, this study presents a solution to this issue by enabling precise control over the distribution of CO2RR products through manipulation of the catalyst’s state. By fine-tuning the catalyst’s structure, researchers can now determine which structural features are responsible for the production of specific reaction products, paving the way for more efficient and targeted production processes.
The key to controlling the size and structure of the catalyst particles lies in the use of alternating electrical pulses. By applying a negative (cathodic) potential, researchers can drive CO2 conversion while inducing the formation of copper nanoparticles. Subsequently, a positive (anodic) potential pulse reverses this process, breaking down the nanoparticles into single atoms once again. Through the manipulation of pulse duration, scientists can guide the formation of nanoparticles and dictate whether the catalyst exists predominantly as single atoms, ultrasmall clusters, or larger metallic nanoparticles, each tailored for different CO2RR products.
To monitor and adjust the catalyst’s transformation in real-time, the research team employed operando quick X-ray absorption spectroscopy, an advanced synchrotron-based technique. This cutting-edge method enables scientists to observe the catalyst’s structural changes during the reaction with sub-second time resolution, ensuring optimal conditions for the desired CO2RR products. By gaining insights into the behavior of the catalyst and the dramatic structural transformations it undergoes during operation, this study sheds light on the intricate process of CO2 reduction and highlights the critical role of catalyst structure in driving the reaction.
While this research showcases the potential applications of this technology in greenhouse gas reduction and the production of green chemicals and fuels, its true significance lies in its contribution to scientific inquiry and the advancements it sets the stage for in the field. By elucidating the mechanisms of catalyst behavior and the impact of structural control on the CO2RR process, this study opens up new avenues for future research and innovation in the realm of climate change mitigation. As we navigate towards a sustainable future, breakthroughs like these offer hope and inspiration for a world where science and technology play a pivotal role in combating the challenges of climate change.

Leave a Reply