The immune system plays a crucial role in protecting the body against pathogens and abnormal cells such as cancer cells. One of the key mechanisms by which the immune system identifies and eliminates these threats is through the presentation of antigens on the surface of cells. Antigen processing is a complex and tightly regulated process that involves the breakdown of proteins into smaller peptides and their presentation on major histocompatibility complex (MHC) molecules. Despite significant advancements in our understanding of antigen processing, there are still many unanswered questions regarding the mechanistic details of this process.
In a groundbreaking study published in the journal Angewandte Chemie International Edition, a team of researchers from the University of Frankfurt in Germany has developed a novel “cage” system that allows for the real-time analysis of antigen processing in living cells. This innovative system involves the use of light to release trapped antigens at specific times and locations, providing researchers with a powerful tool to study the dynamics of antigen flux.
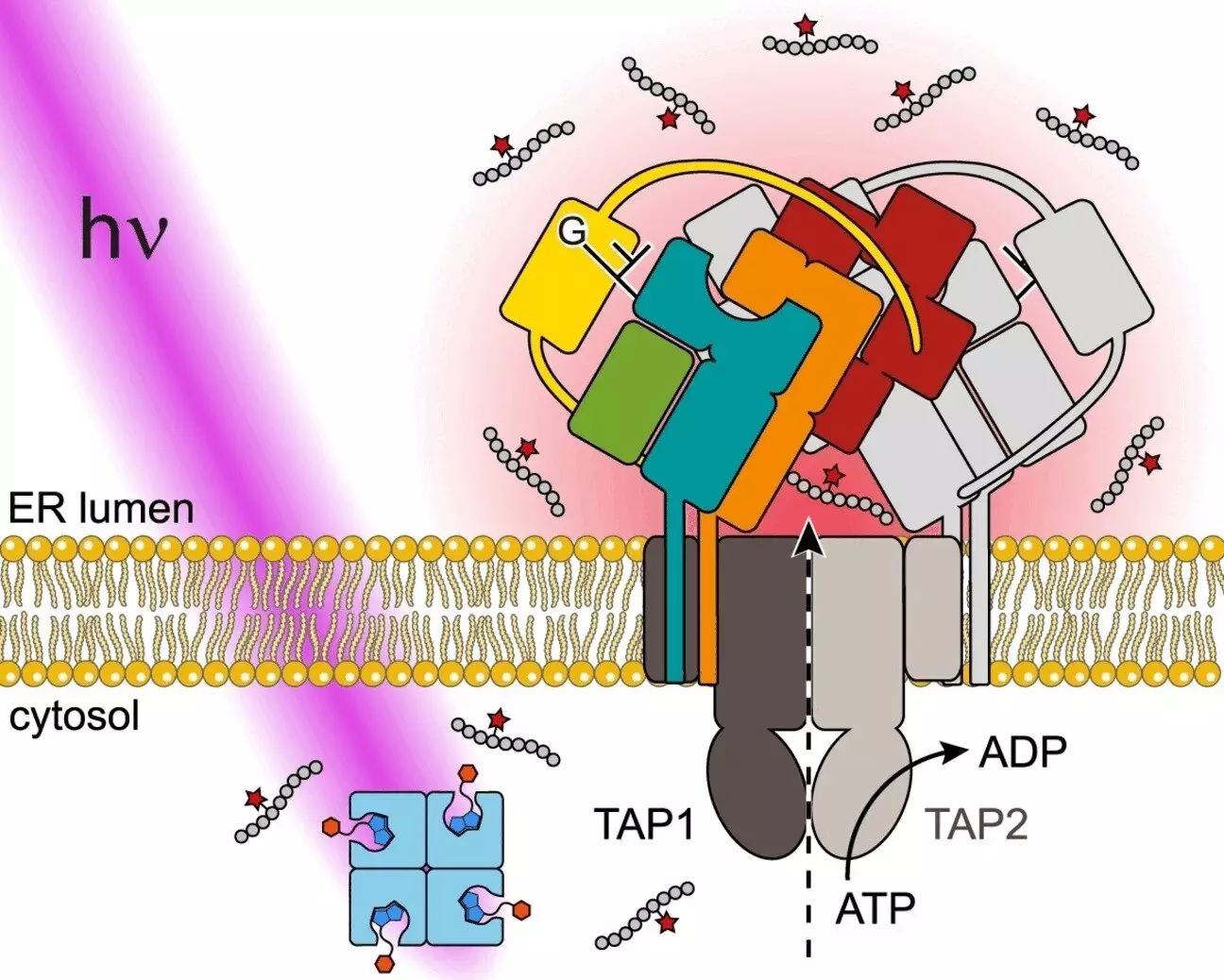
The key feature of this new system is its ability to release antigens from their “caged” inactive state in response to light stimulation. By using a peptide derived from an HIV antigen as a model, the researchers were able to demonstrate the versatility and effectiveness of this approach. The peptide epitope is first bound to a voluminous protein called streptavidin via a linker that can be split apart by light. When exposed to UV light, the peptide epitope is instantly released from its “cage” and is recognized by the antigen processing transporter (TAP), leading to its transport across the endoplasmic reticulum (ER) membrane and loading onto MHC molecules.
This novel system opens up a wide range of possibilities for studying antigen processing and immune surveillance in various biological contexts. By being able to precisely control the release of antigens in living cells, researchers can gain valuable insights into the mechanisms underlying antigen translocation, PLC assembly, and the interaction between different subunits involved in antigen processing. Moreover, the noninvasive nature of light stimulation allows for experiments to be conducted with high precision and spatiotemporal control.
The development of this light-triggered antigen release system represents a major advancement in the field of immunology and antigen processing. By providing researchers with a powerful tool to study the dynamics of antigen flux in real time, this system has the potential to deepen our understanding of the immune response and facilitate the development of novel therapeutic strategies for combating infectious diseases and cancer. As further research is conducted and the capabilities of this system are expanded, it is clear that we are on the brink of a new era in immunology.

Leave a Reply