Minerals and mineralized tissues produced by various organisms have fascinated scientists for centuries. The formation of these intricate structures, such as nacre, is a complex process that involves the extraction of calcium and carbonate ions from water. However, the exact mechanisms and conditions that lead to the formation of nacre and other biominerals remain a topic of intense debate among experts. One crucial player in this process is amorphous calcium carbonate (ACC), a non-crystalline intermediate that has been found to be essential in biomineralization. In a recent study published in Nature Communications, researchers from the University of Konstanz and Leibniz University Hannover have successfully decoded the formation pathway of ACC, shedding new light on the understanding of biomineralization.
Deciphering the Structure of ACC
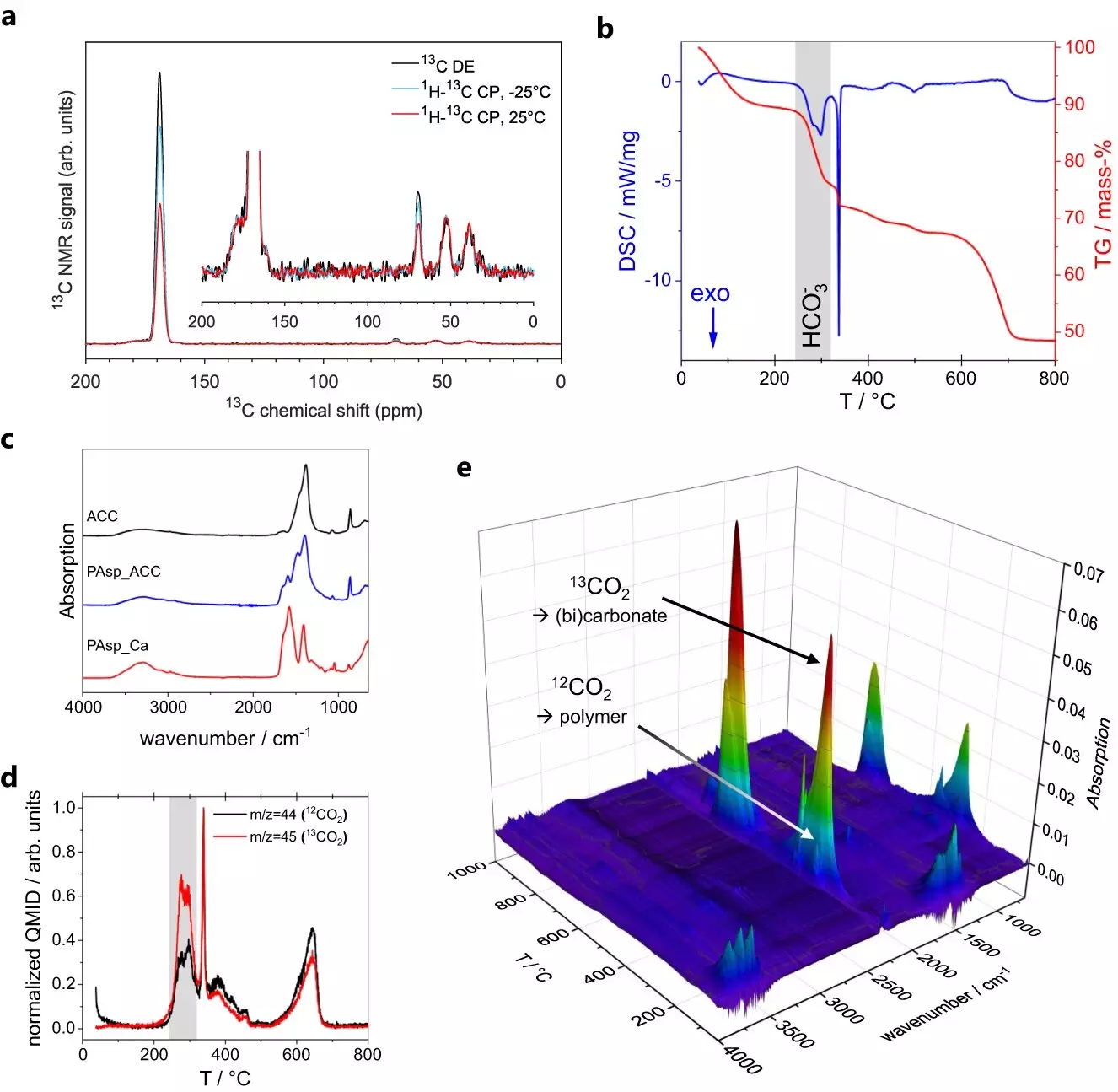
In their quest to unravel the mysteries of ACC formation, the research team led by Denis Gebauer of Leibniz University Hannover and Guinevere Mathies of the University of Konstanz utilized advanced techniques such as magic-angle spinning nuclear magnetic resonance (MAS NMR) spectroscopy. By analyzing tiny ACC particles, the team aimed to determine their structure. However, interpreting the spectra of ACC proved to be a challenge initially. The dynamics observed in the spectra were difficult to model, indicating the need for further investigation.
Intriguingly, Maxim Gindele from the Gebauer group discovered that ACC exhibits electrical conductivity. To study this property, the researchers employed conductivity atomic force microscopy (C-AFM). This technique involved using a minuscule cantilever to scan the surface of ACC particles and measure their conductivity through a current passing through the tip of the cantilever. The observation of conductivity provided a valuable clue in understanding the dynamics of ACC.
Building upon the discovery of conductivity, Sanjay Vinod Kumar of the Mathies group conducted further MAS NMR experiments to investigate the dynamics within ACC. These experiments led to the identification of two distinct chemical environments within the ACC particles. The first environment involved rigid calcium carbonate structures, where water molecules could only undergo 180-degree flips. The second environment consisted of water molecules experiencing slow tumbling and translation, accompanied by the presence of dissolved hydroxide ions.
Reconciling the Environments and Conductivity
The researchers faced the challenge of reconciling these two distinct environments with the observed conductivity. Solid salts are typically insulators, suggesting that the mobile water environment in ACC played a critical role. In their new model, the team proposed that the mobile water molecules form a network throughout the ACC nanoparticles, while the dissolved hydroxide ions carry the charge. The formation of the two chemical environments was attributed to the tendency of calcium and carbonate ions to form pre-nucleation clusters in water. These clusters then undergo phase separation and coalesce into dense, liquid droplets. The rigid, less mobile environment found in ACC stems from the core of these droplets, while the network of mobile water molecules remains from imperfect coalescence during dehydration towards solid ACC.
Implications and Future Applications
The deciphering of ACC’s formation pathway marks a significant milestone in the understanding of biomineralization. It not only brings us closer to unlocking the secrets behind the intricate structures found in nature but also has potential applications in various fields. The knowledge gained from this research could aid in the development of cementitious materials capable of binding carbon dioxide, contributing to sustainable construction practices. Additionally, the discovery that ACC exhibits electrical conductivity opens up possibilities for its use in electrochemical devices.
The research conducted by the scientists from the University of Konstanz and Leibniz University Hannover has shed new light on the formation pathway of amorphous calcium carbonate. Through the utilization of advanced techniques and careful analysis, the team deciphered the structure of ACC and revealed its two distinct chemical environments. These findings not only provide crucial insights into the process of biomineralization but also have potential applications in various scientific and technological fields. As our understanding of the intricacies of biomineralization deepens, the possibilities for future discoveries and innovations continue to expand.


Leave a Reply