Microplastics have increasingly become a focal point in environmental research due to their pervasive presence in aquatic ecosystems. These tiny fragments often originate from a variety of sources, including the breakdown of larger plastic items and the shedding of synthetic fibers from textiles. Their accumulation poses significant risks to wildlife and human health since they can absorb toxic substances and facilitate their entry into the food chain. Emerging research sheds light on a less-discussed aspect of microplastics: their behavior in freezing conditions and the subsequent melting process, which may alter their environmental impact profoundly.
Recent findings, documented in the journal Environmental Science & Technology, reveal that freezing can modify the characteristics of microplastics in individual water bodies. When ice forms on water, microplastics can become trapped beneath, and as temperatures rise, the melting ice may lead to alterations in the size and buoyancy of these particles. The study, conducted by Chunjiang An and his colleagues, specifically examines how different types of polymers—such as polyethylene (PE), polyurethane (PU), and polytetrafluoroethylene (PTFE)—respond to freezing and thawing in both freshwater and saline environments.
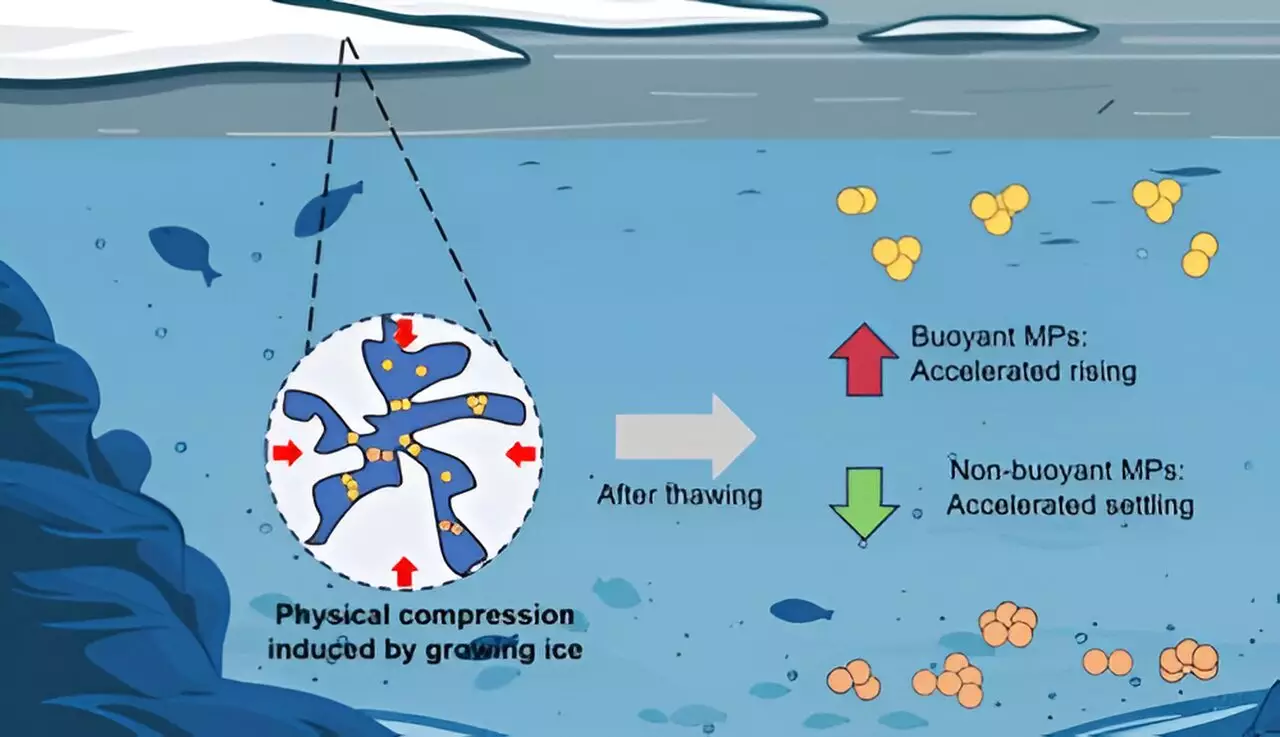
This research highlights a pivotal observation: while PE tends to float due to its lower density than water, other polymers like PU and PTFE are denser and exhibit a tendency to sink. The ability of these particles to remain suspended or to settle on the seabed is influenced not just by density but also by environmental factors including salinity, temperature, and the physical interactions of multiple particles in a given fluid.
The researchers’ laboratory experiments revealed intriguing dynamics in microplastic behavior post-freezing. Upon examining particle size, the study indicated that all types of microplastics—when frozen—experienced an increase in size upon thawing, although the variations were distinct among polymers. PE particles exhibited a 46% increase, while PU showed a meager 9% increase. This discrepancy raises compelling questions about how the molecular structure and hydrophilic or hydrophobic nature of these polymers govern their behavior in aquatic systems.
Moreover, the study proposes that in saline solutions, the freezing process does not influence particle size, likely due to the presence of brine channels within the ice matrix. These channels allow microplastics to navigate around each other, thus preventing the formation of larger aggregates that could otherwise settle.
An essential component of the research was the investigation of the forces at play that dictate the movement of microplastics after ice thaw. The balance of gravitational force, buoyancy, and drag was meticulously calculated to determine how quickly microplastics would settle or remain suspended in water. The study’s revelations suggest that after the freeze-thaw cycles, PE particles may rise faster while PU and PTFE particles would settle more rapidly. Such findings offer insights into how microplastics could disperse in colder climates and enter sediment layers in freshwater lakes and rivers.
Despite the findings, it is critical to recognize the short duration of freezing applied in these experiments, which was substantially less than typical durations observed in natural settings. In ecosystems where ice persists for extended periods, the implications for microplastic behavior could differ significantly, warranting further investigation.
With climate change leading to altered freezing patterns and altered aquatic environments, understanding the fate of microplastics in ice-covered waters is increasingly pressing. The research underlines a fundamental principle that changes in environmental conditions, such as freezing, could significantly influence the ecological consequences of microplastics in water bodies.
This pioneering research provides a foundational understanding of how microplastics behave under the influence of freezing and thawing, setting the stage for further exploration into the ecological impacts of these materials. As microplastics continue to infiltrate vital ecosystems, it is crucial for scientists to expand upon this work, shedding light on their long-term implications on environmental health and water quality. By delving deeper into the dynamics of microplastics, researchers can better inform policies and practices to mitigate their effects, ensuring the protection of our aquatic environments for generations to come.

Leave a Reply