The study published in Nature Communications by RIKEN scientists has shed light on the intricate process of water molecules losing energy at the interface with air. This new discovery provides a more profound understanding of the dynamics that take place at water surfaces, revealing the complexity of hydrogen bonding and relaxation mechanisms.
Water, a substance vital for life, exhibits numerous anomalous properties that set it apart from other liquids. One of these peculiarities is its unusually high freezing and boiling points, coupled with the fact that ice is less dense than liquid water. These anomalies can be attributed to the presence of hydrogen bonds between neighboring water molecules, formed due to the unequal attraction of electrons by oxygen and hydrogen atoms.
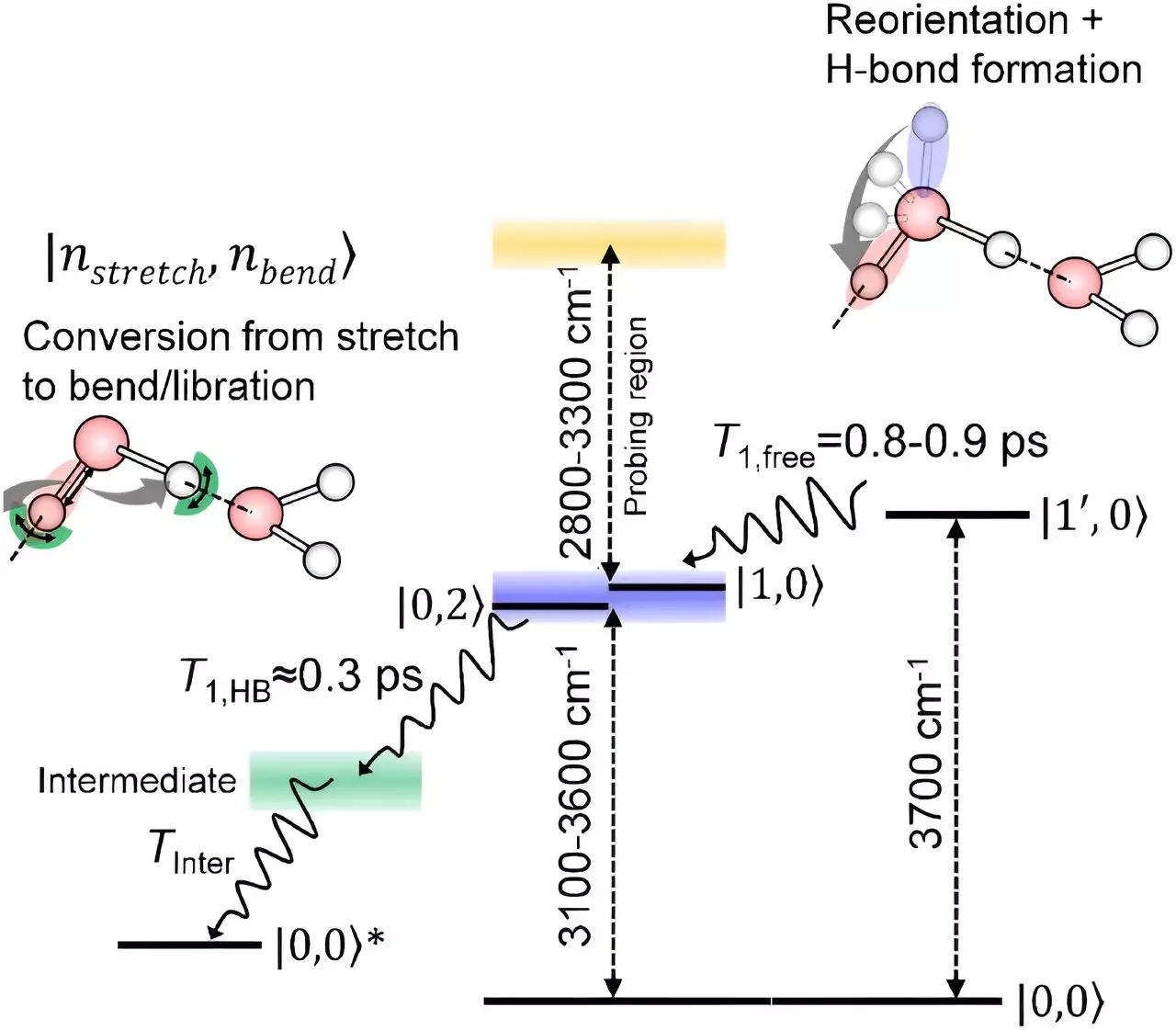
Among the countless water molecules in a body of liquid, those on the surface experience hydrogen bonding in a distinct manner. Unlike their counterparts in the bulk, surface water molecules have a portion that protrudes into the air without forming typical hydrogen bonds. Understanding how these surface molecules relax after being stretched has been a challenge for scientists, as isolating their signals is a complex task.
The Innovation in Spectroscopy Techniques
To address this knowledge gap, Tahei Tahara and his team at the RIKEN Molecular Spectroscopy Laboratory have pioneered sophisticated spectroscopy techniques over the past decade. Their latest development involves utilizing infrared spectroscopy to observe the relaxation of oxygen-hydrogen bonds in surface water molecules with unprecedented sensitivity. This innovative approach has enabled the team to track the rotational and relaxation movements of these bonds accurately.
A Comprehensive Insight into Relaxation Processes
Through their experiments, the researchers discovered that oxygen-hydrogen bonds at the surface initially undergo rotation without losing energy before transitioning into a relaxation phase resembling that of interior water molecules. This similarity in relaxation mechanisms between surface and bulk water molecules highlights the interconnected nature of hydrogen bonding dynamics, regardless of the molecular location within the liquid.
The insights gained from this study not only enhance our comprehension of water’s behavior at interfaces but also provide a foundation for investigating chemical reactions occurring at the water-air boundary. Tahara and his team plan to further utilize their spectroscopic technique to explore the intricacies of surface reactions and elucidate the role of water molecules in catalytic processes.
The research conducted by RIKEN scientists offers a comprehensive portrayal of the behavior of water molecules at the surface interface, unraveling the complexities of hydrogen bonding and relaxation dynamics. This study not only deepens our understanding of fundamental water properties but also paves the way for future explorations into surface chemistry and interfacial reactions.

Leave a Reply