Fuel cells have long been hailed as a promising source of green energy, offering the potential to revolutionize various industries such as transportation and power generation. These cells produce electricity through a chemical reaction that ultimately produces water and heat as byproducts, making them environmentally friendly. While much of the research in this area has focused on cells that use platinum as the catalyst, there are limitations to this approach. Platinum is scarce, expensive, and not particularly stable for this application. However, a recent study conducted by researchers from Western University has unveiled a breakthrough that integrates other metals, namely palladium and cobalt, with platinum. This integration reduces the need for platinum and creates a more stable catalyst for fuel cells.
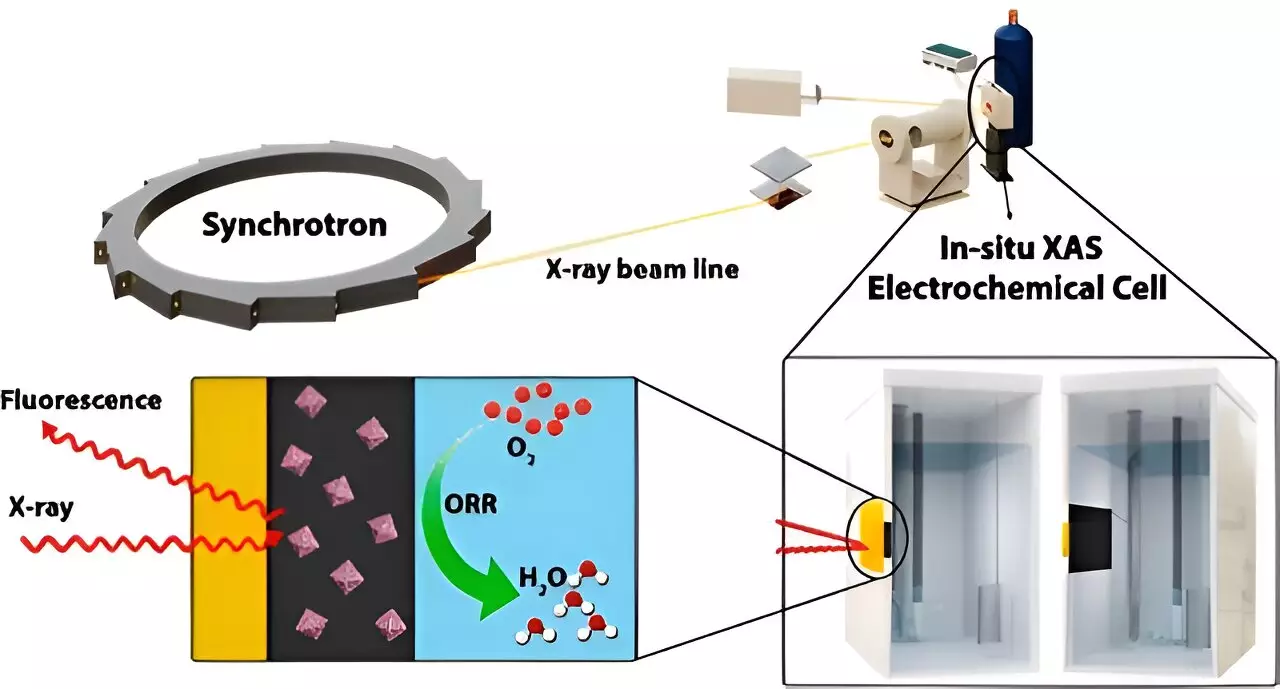
Tsun-Kong (T.K.) Sham, Xueliang (Andy) Sun, Ali Feizabadi, and their collaborators from Western University’s Department of Chemistry and Department of Mechanical & Materials Engineering embarked on a quest to improve fuel cell technology. They utilized the Canadian Light Source (CLS) at the University of Saskatchewan (USask) to develop and test their innovative approach. The CLS allowed them to analyze the new nanomaterials in real-time, providing valuable insights into the binding of oxygen with platinum and the impact of electron transfer between platinum and other metals in the catalyst. This analysis shed light on the catalyst’s efficiency and performance, enabling the researchers to enhance the platinum’s catalytic performance and improve the overall durability of the catalyst.
By integrating palladium and cobalt with platinum, the researchers effectively reduced the reliance on scarce and expensive materials. Additionally, this integration enhanced the efficiency and lifespan of proton-exchange membrane fuel cells (PEMFCs). The findings of this study have the potential to make fuel cells more economically viable and environmentally friendly, which could drive the broader adoption of clean energy technologies. The stability and positive results produced by the novel approach pave the way for the commercialization of an alternative energy source, marking a significant step toward sustainable energy solutions.
The breakthrough achieved by the team at Western University opens up new possibilities for the advancement and commercialization of fuel cell technology. The integration of palladium and cobalt with platinum not only reduces costs but also addresses the limitations of fuel cells by increasing their efficiency and improving their environmental impact. It is evident that further research is needed to refine and optimize this approach for mass production. However, the initial results are highly promising and provide a strong foundation for future developments in the field of fuel cells.
The integration of palladium and cobalt with platinum in fuel cell technology is a significant step toward the sustainable and widespread use of clean energy. This breakthrough, achieved by researchers from Western University, offers a solution to the scarcity, cost, and instability issues associated with platinum catalysts. By reducing the reliance on platinum, the commercial viability of fuel cells is increased. Moreover, by enhancing the catalytic performance and durability of the catalyst, the overall efficiency and lifespan of PEMFCs are improved. The positive results obtained from the study mark an important milestone and pave the way for the broader adoption of clean energy technologies. As further research and development are conducted, the integration of alternative metals in fuel cells has the potential to transform various industries and contribute to a greener and more sustainable future.

Leave a Reply