In a recent study, researchers from the University of Münster have developed a groundbreaking strategy for converting carbon-nitrogen atom pairs in commonly used pyridines into carbon-carbon atom pairs. This innovative method has significant potential in the search for new active ingredients for drugs and materials. The study, led by Professor Armido Studer from the Institute of Organic Chemistry, was recently published in Nature Chemistry.
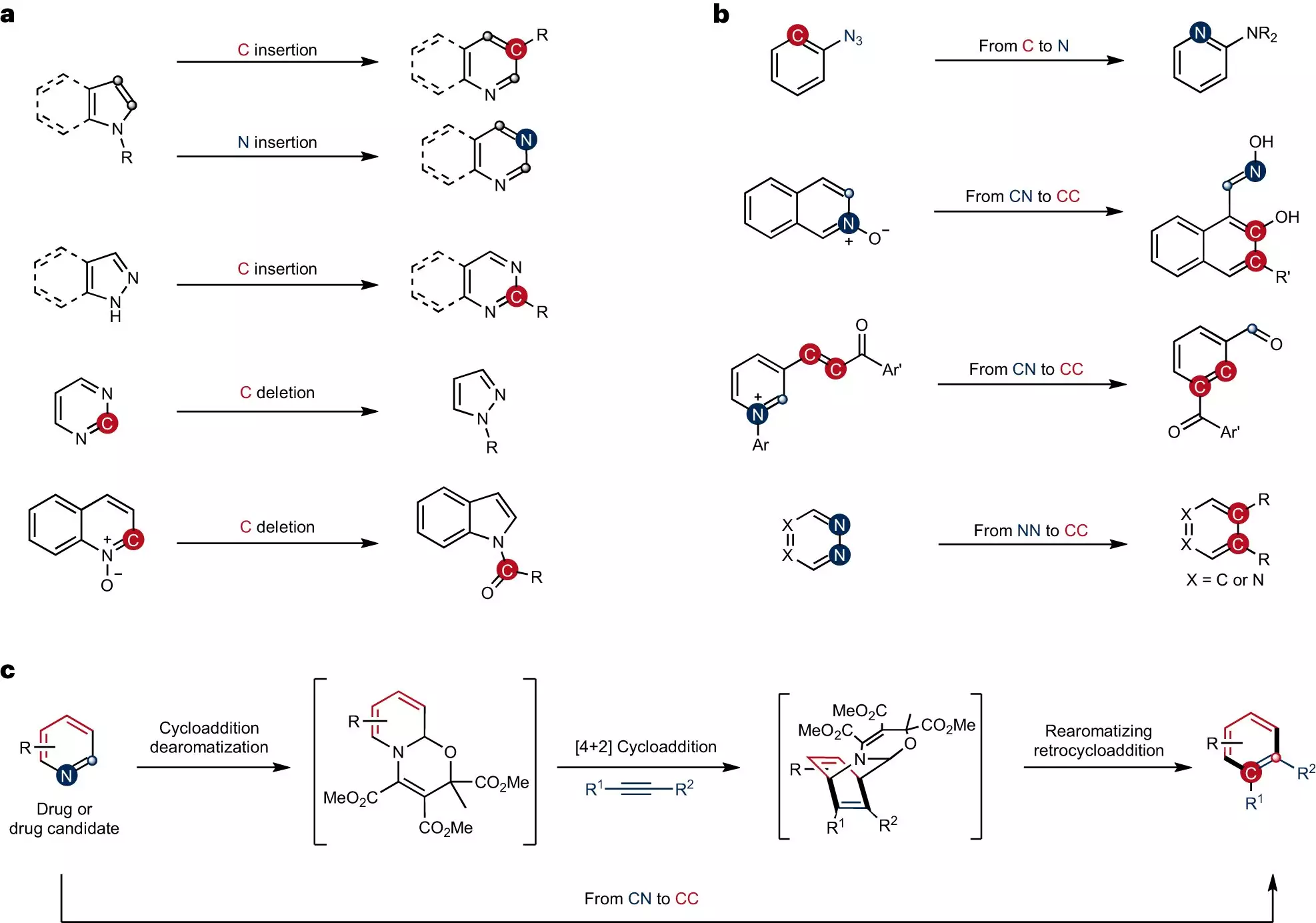
Skeletal editing is a well-known technique in the field of chemistry, used to precisely modify ring-shaped structures by replacing individual atoms. However, the conversion of carbon-nitrogen atom pairs in pyridines, a frequently employed ring-shaped compound, into carbon-carbon atom pairs has remained a challenge. Professor Studer’s team has successfully developed a new strategy that addresses this hurdle.
Unlike peripheral functionalization, which involves attaching groups of atoms to the ring structure, skeletal editing requires the cleavage of robust bonds between carbon atoms or between a carbon atom and another atom within the ring. This process can be compared to a surgical procedure in organic synthesis. Up until now, no synthesis strategy existed for swapping complex pyridines using skeletal editing.
To overcome the inert nature of the pyridines, the team embarked on modifying their bonding structure through dearomatization. This step created significantly more reactive intermediates, enabling the subsequent cycloaddition and rearomatization processes necessary for the formation of the skeletal-edited compounds. The team successfully produced benzenes and naphthalenes with functional groups precisely attached to specific positions, establishing a new pathway for introducing synthetically valuable and medically significant functional groups onto ring structures.
One notable achievement of this study is the development of a one-pot procedure for the synthesis. In a one-pot reaction, all the reagents required react with one another within a single vessel, simplifying the process and reducing the need for multiple reaction steps. This not only improves efficiency but also makes the approach more accessible for wider applications in the field of organic chemistry.
Dr. Christian Mück-Lichtenfeld, who contributed to the study from the Institute of Organic Chemistry, conducted a theoretical analysis of the reaction sequence’s mechanism. This analysis provides valuable insights into the fundamental understanding of the conversion of carbon-nitrogen atom pairs to carbon-carbon atom pairs, paving the way for further advancements in the field.
The development of a new strategy for converting carbon-nitrogen atom pairs into carbon-carbon atom pairs, as demonstrated by the team from the University of Münster, represents a significant breakthrough in the field of organic chemistry. The ability to apply skeletal editing to pyridines opens up possibilities for the discovery of new drugs and materials. This innovation not only expands the synthetic toolbox available to chemists but also highlights the importance of continuous research and innovation in the pursuit of scientific advancements.

Leave a Reply