Solar energy has become a popular source of sustainable energy in recent years. However, the intermittent nature of solar energy has limited its use for certain applications, such as IoT devices, live remote sensing, and off-grid power supplies. Conventionally, batteries are used to store the energy generated by solar cells, but combining the two technologies requires separate packaging, making it cumbersome to install and increasing the cost and ohmic losses in the device. Moreover, these physical limitations make the system bulky, limiting its potential applications.
The Solution: Photorechargeable Batteries (PRBs)
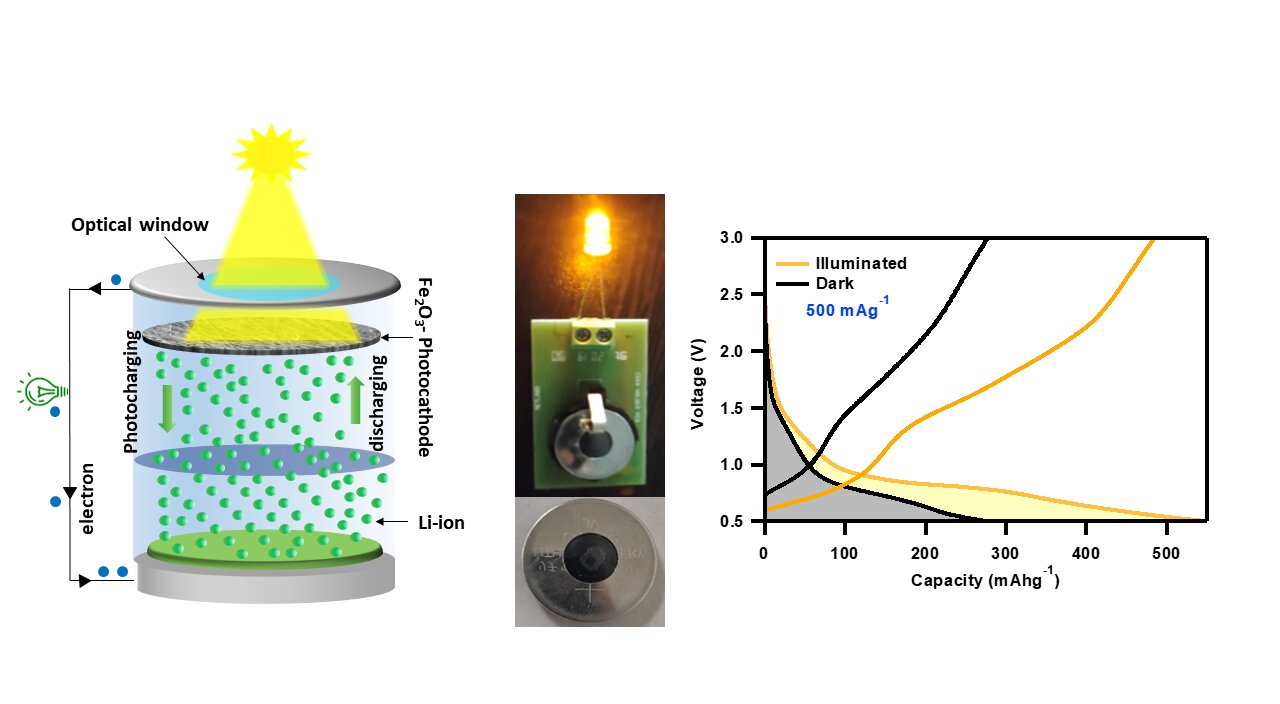
Researchers at the Advanced Energy Materials Lab at the Indian Institute of Technology Jodhpur have developed a promising solution to these limitations: photorechargeable batteries (PRBs). PRBs can perform solar energy harvesting and storage simultaneously using advanced nanomaterials, making them lightweight and efficient compared to conventional combinations of PVs and batteries. In their study published in Advanced Sustainable Systems, the researchers demonstrated that iron oxide nanorods can work as an active material to form efficient and low-cost photocathodes for PRB applications.
How It Works
Iron oxide nanorods can simultaneously harvest solar radiation in the visible region due to their bandgap of ~2.1 eV and store Li-ions efficiently. The highly nanoporous photocathodes are made using hematite, C-61 carbon (PCBM), and carbon nanotubes. Hematite absorbs sunlight and produces photogenerated charge carriers, while PCBM and carbon nanotubes conductive additives provide a suitable pathway for photogenerated electrons to reach the current collector and initiate photocharging. The PRB showed independent charging when illuminated with a 470 nm blue LED, achieving a significant photoconversion and storage efficiency (PCSE) of 1.988%.
Fe2O3 nanorods absorb photons of energy higher than their energy band gap and generate photogenerated charge carriers at the photocathode. The conductive additives provide a favorable pathway for photoelectrons to reach the current collector and further the anode through an external circuit. Meanwhile, photoholes present in Fe2O3 oxidize Fe0 to Fe3+, which provides repulsion to the Li+ toward the Li-metal anode via the electrolyte. As a result, the Li-ions are reduced at the anode to form Li-metal, resulting in photocharging.
This technology could be beneficial for continuous operation of sensors, allowing PRBs to charge during daylight hours and use stored energy to power devices at night or when there is no sunlight. The PRB was able to power a commercial 3V LED even after three months of fabrication, indicating that the demonstrated iron oxide nanorods-based PRB is highly stable and does not suffer from self-discharge issues.


Leave a Reply