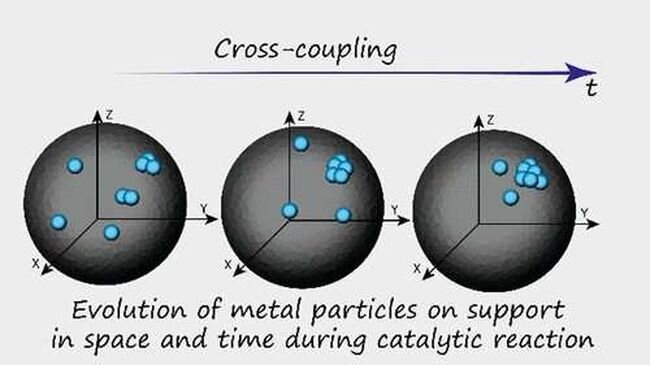
A team of chemists from the Russian Academy of Sciences has found that metal atoms, not nanoparticles, play a crucial role in catalysts used in fine organic synthesis. The study, published in the Journal of the American Chemical Society, utilized various types of electron microscopy to track a catalyst’s region during a reaction to understand its progress.
Limitations of Traditional Approaches
Before this study, chemists used two methods to examine reactions. The first is the most basic: observe and/or measure the reaction as ingredients are added. This approach can be facilitated using high-speed cameras, but it cannot be used with reactions that occur in the nanoscale. In such cases, chemists must use the second method: capturing the states of all components before and after the reaction and comparing them to understand better what happened. However, this approach has limitations, and there is no way to prove that the objects under study correspond with one another.
Methodology and Results
Chemists have been working on a new approach in recent years: monitoring the action of a single particle during the reaction. This method has limitations as well, and it cannot be used for reactions that occur in the nanoscale. The researchers used multiple types of electron microscopy and machine-learning algorithms to test their ideas in this new effort. They studied reactions using a catalyst with embedded palladium nanoparticles and a carbon substrate. By utilizing various types of electron microscopes and training a machine-learning algorithm with the outcomes, they tracked a catalyst’s region during a reaction and found that individual metal atoms and clusters, in addition to nanoparticles, play a role in the reaction. According to further study, nearly 99% of the catalytic activity was due to the palladium atoms, not the nanoparticles, despite the nanoparticles accounting for just 1% of the palladium mass.

Leave a Reply