Samarium, a rare earth element, has carved a niche in the field of organic chemistry due to its unique capacity for facilitating single-electron transfer reactions. Among its various compounds, samarium iodide (SmI2) stands out for its stability and efficacy at ambient temperatures, making it an essential reagent in synthesizing pharmaceuticals and bioactive compounds. However, traditional methodologies often demand significant amounts of SmI2, alongside harsh chemicals, rendering operations laborious and economically burdensome.
The high costs associated with samarium, combined with the resource-heavy nature of existing methods, underscore a pressing need for more sustainable and efficient catalytic systems. To address this, chemists have explored alternatives aimed at reducing the reliance on samarium reagents to catalytic concentrations. Nevertheless, these efforts frequently encounter challenges, requiring either harsh reaction conditions or expensive reducing agents that still consume substantial samarium, often comprising 10-20% of the overall reactants.
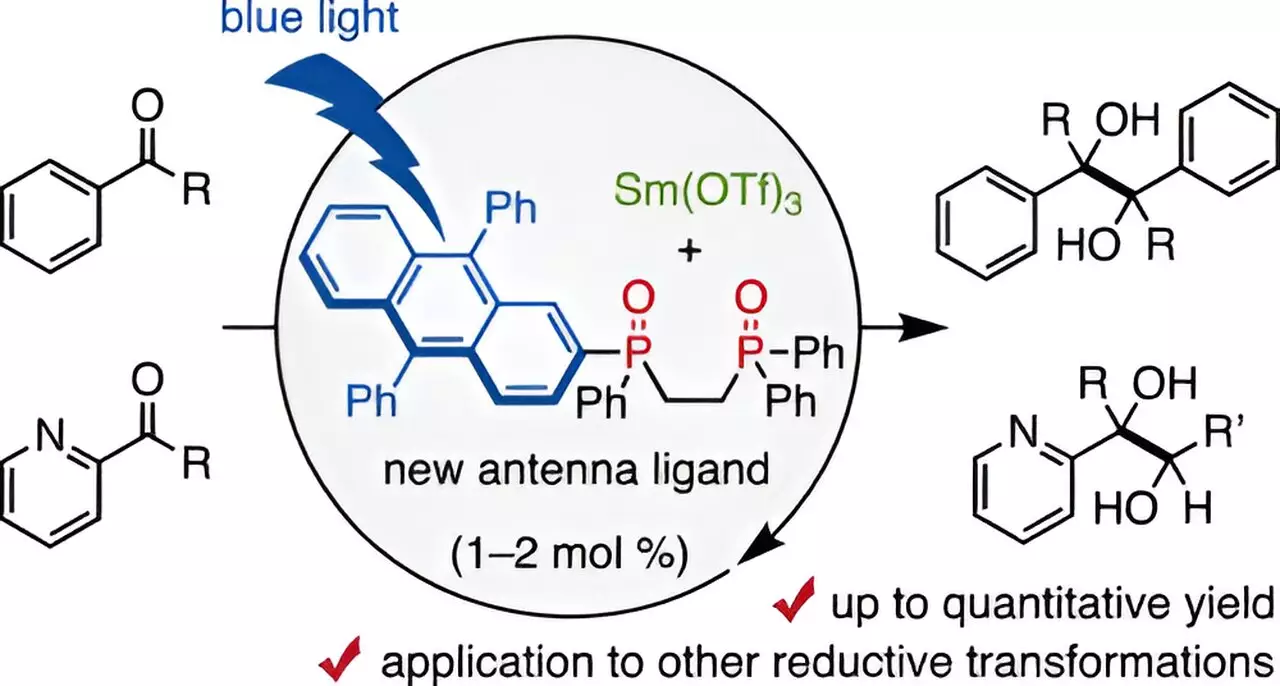
Recently, an innovative approach emerged from a research team at Chiba University, led by Assistant Professor Takahito Kuribara. Their research has unveiled a transformative method utilizing a bidentate phosphine oxide ligand, specifically designed to coordinate with trivalent samarium. This groundbreaking ligand, substituted with 9,10-diphenyl anthracene (DPA), serves as a visible-light antenna, enabling the leverage of low-energy visible light in samarium-catalyzed reactions.
Kuribara notes that the incorporation of antenna ligands, which enhance the excitation of lanthanoid metals like samarium, marks a significant advancement in the chemistry toolkit. Following up on their prior work on a DPA-substituted secondary phosphine oxide that facilitated visible-light-driven reduction-oxidation reactions, the team designed this new ligand with a focus on enabling the efficiency of samarium catalysts while dramatically reducing the quantity of samarium needed.
The research team conducted a comprehensive series of experiments demonstrating the remarkable effectiveness of their visible-light antenna in catalyzing pinacol coupling reactions among aldehydes and ketones—a process integral to pharmaceutical synthesis. The findings were nothing short of transformative: using merely 1-2 mol% of the samarium catalyst led to impressive yield rates, reaching up to 98%, a stark contrast to traditional methodologies. Moreover, these reactions could successfully take place using milder organic reducing agents, such as amines, rather than the highly reactive variants commonly employed in the past.
An intriguing aspect of their findings revealed that the judicious addition of water could enhance reaction yields, although excessive water had the opposite effect. To dissect the efficacy of the DPA-1 ligand, the researchers explored the interaction characteristics between the samarium catalyst and the ligand. They established that DPA-1 serves as a multifunctional ligand, proficient in coordinating with samarium, selectively absorbing blue light, and efficiently transferring electrons to the metal center.
The versatility of the samarium catalyst combined with DPA-1 was evident, as it facilitated various molecular transformations, including crucial carbon-carbon bond formations and cleavage reactions, all fundamental to drug development. The researchers also highlighted the potential of integrating samarium-catalyzed reductions with photo-oxidation processes, harnessing visible light as a renewable energy source.
The advancements brought forth by Kuribara and his team not only showcase a significant reduction in the requisite amounts of samarium for effective catalysis but also position trivalent samarium, known for its superior stability, as a preferable alternative to its divalent counterpart. This innovative visible-light antenna ligand paves the way for new methodologies in organic synthesis, ultimately guiding the development of more efficient and sustainable synthetic protocols.
As the scientific community continues to mandate greener chemistry, the study underscores the importance of innovative catalyst designs. By utilizing minimal amounts of samarium under milder conditions, researchers may enhance the sustainability of chemical manufacturing, particularly in areas as critical as drug development. The findings, published in the Journal of the American Chemical Society, represent not just an incremental step but a significant leap forward in samarium-based catalysis and organic chemistry, addressing urgent economic and environmental concerns in the field.

Leave a Reply