The proliferation of synthetic polymers, particularly since the 1950s, has ushered in a double-edged sword. On one hand, these materials have brought convenience and innovation into everyday life; on the other, they have created an environmental crisis due to the staggering 8.3 billion metric tons of polymers produced globally—most of which remain in landfills or incinerators. With only a meager 600 million metric tons recycled, the prevailing waste management methods leave much to be desired. The indestructible nature of these materials, especially polyolefins, decidedly makes tackling this issue both critical and complex.
The Research Breakthrough
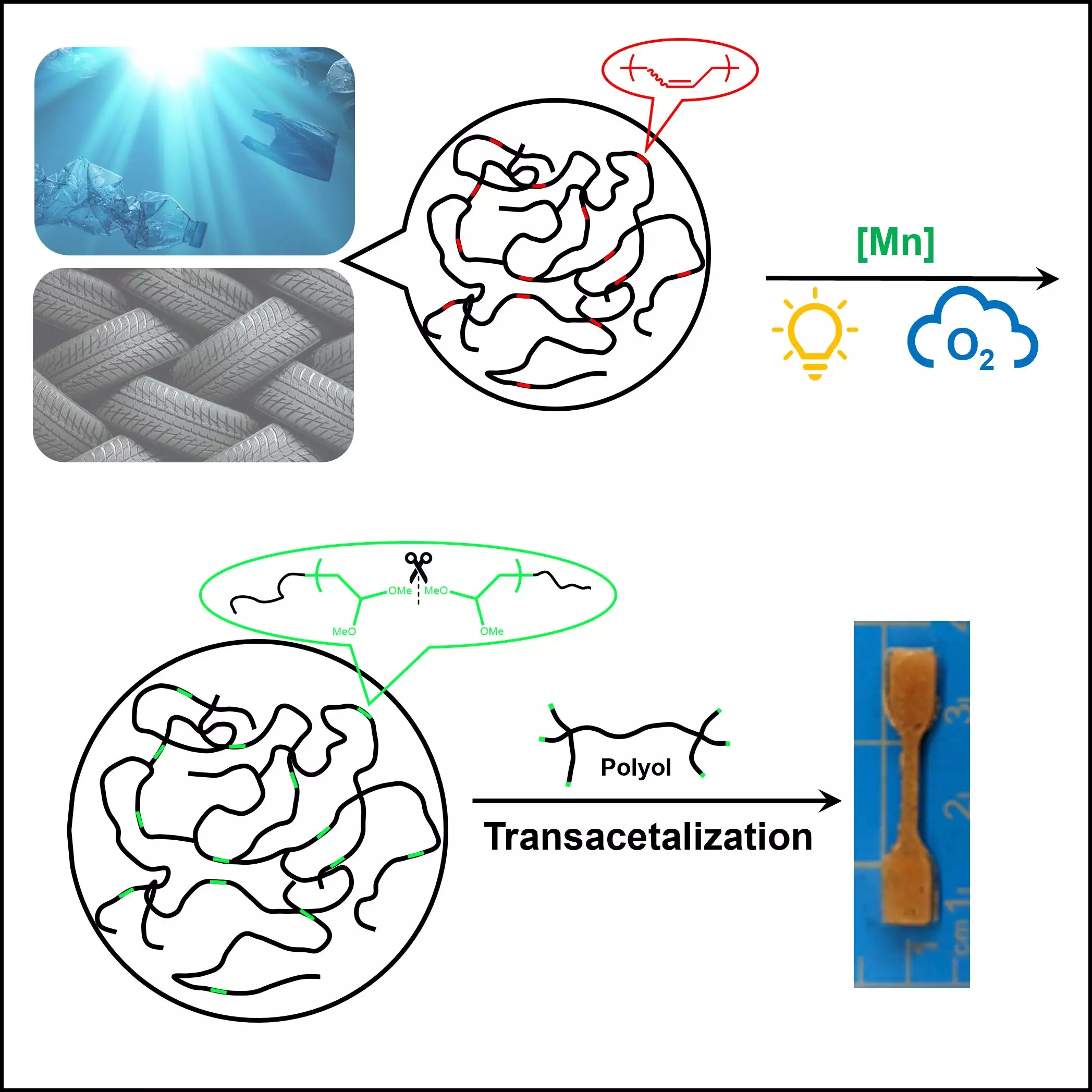
In a compelling study detailed in Cell Reports Physical Science, Dr. Junpeng Wang and his team from The University of Akron have unveiled a fresh methodology that could transform polymer recycling as we know it. The researchers leveraged the inherent properties of oxygen and light to catalyze the breakdown of unsaturated polymers, such as rubber and plastics. This novel approach circumvents the limitations of traditional recycling techniques, which often rely on harsh chemical processes that require significant energy input and create toxic by-products, further worsening the environmental footprint.
The Science of Change
At the core of this innovative research is the idea of enhancing the reactivity of polymers by introducing unsaturation. This fundamentally alters the polymers’ structure, making them more amenable to environmental degradation. Traditional oxidative processes like ozonolysis have faced criticism for their inefficiency and environmental detriment. However, Dr. Wang’s approach embraces oxygen as a green oxidant—a readily available and eco-friendly alternative that, until this breakthrough, suffered from slow reaction rates and lack of control during degradation. The prowess of this new method lies in activating a catalyst using light, which enables the breakdown of polymers at room temperature and atmospheric pressure, drastically reducing energy costs and enhancing scalability.
Addressing Environmental Challenges
The implications of this research extend far beyond the technical aspects of recycling. By providing a controlled and efficient means of polymer degradation, this study offers a vital lifeline in the battle against plastic pollution. As public awareness grows regarding environmental sustainability, innovative strategies such as Wang’s are necessary for industries to adapt and evolve. The ability to recycle unsaturated polymers effectively can significantly reduce pollution and decrease the dependency on virgin plastic production.
A Call to Action
This transformative approach championed by Dr. Wang and his team must not only be celebrated but also rapidly implemented across industries. Policymakers should take note; fostering a research-friendly environment and encouraging partnerships between academic institutions and industries could catalyze the widespread adoption of such sustainable practices. Focusing on environmentally sound methods of recycling isn’t just a solution but a necessity if we are to curtail the devastating impact of plastic pollution that threatens our ecosystems and health. As innovators like Wang pave the way for sustainable solutions, it is crucial to ensure that their discoveries are translated into action, shaping a cleaner, greener future for generations to come.

Leave a Reply