In today’s rapidly developing world, pollution remains a critical concern, particularly with the rising levels of micropollutants—substances that are present in minute quantities but can cause substantial environmental damage. Micropollutants include a variety of trace chemicals, such as pesticides and pharmaceuticals, which can infiltrate our water systems, leading to dire consequences for both wildlife and human health. Tackling this issue requires innovative solutions that harness advanced scientific principles, and recent research has introduced promising methodologies to mitigate this problem effectively.
Innovative Photocatalysis: A Game Changer in Wastewater Treatment
One such breakthrough is in photocatalysis, a process that utilizes sunlight and specialized materials to break down harmful pollutants. At the heart of this procedure are semiconducting nanomaterials, particularly titanium dioxide (TiO2), which are enhanced with noble metal co-catalysts like gold. This combination has demonstrated a remarkable potential to adsorb and degrade toxic substances on their surfaces, showcasing a safe and sustainable mechanism for water purification. The recent study led by a team at Cornell University sheds light on the intricate interactions between these materials and illustrates how they can be optimized for maximal efficiency.
The Synergistic Power of Gold and Titanium Dioxide
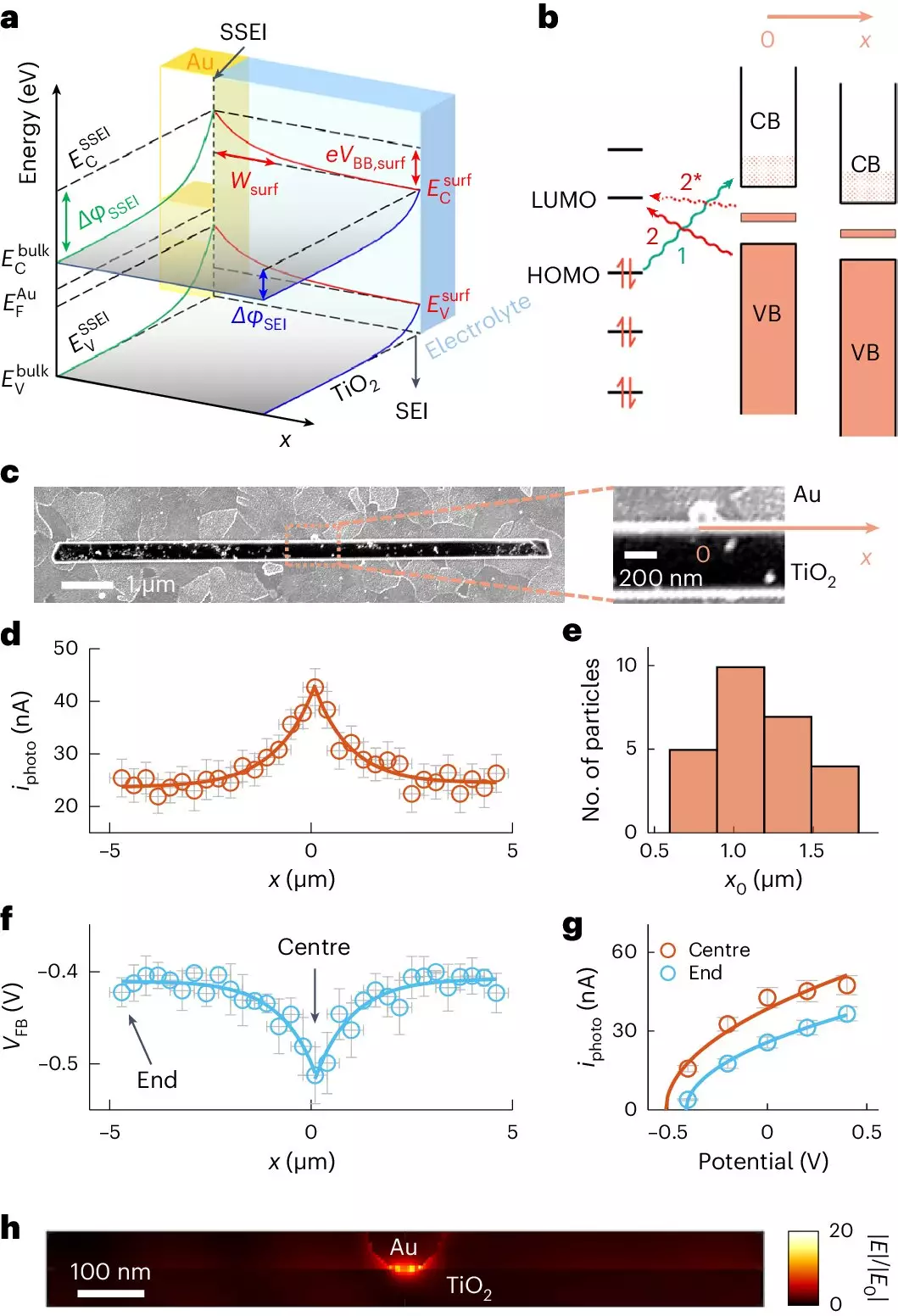
The inclusion of gold nanoparticles as co-catalysts is not an arbitrary choice; this pairing has been widely adopted due to its proven efficacy. However, the research team uncovered new revelations regarding the mechanics of adsorption—how molecules classify and adhere to surfaces. Utilizing a novel imaging technique known as adCOMPEITS, they explored how gold nanoparticles can enhance the adsorption of pollutants far beyond their immediate vicinity—up to 10 times further than previously observed. By employing a dual-probe system that uses fluorescent indicators, researchers effectively mapped the competition between toxic micropollutants and the fluorescent molecules, revealing intricate details about their behavior on the TiO2 surface.
Groundbreaking Findings on Surface Band Bending
Among the pivotal discoveries was the phenomenon of “surface band bending.” This term refers to the alteration of electronic properties on the TiO2 surface due to the presence of gold nanoparticles, which affects how substances adhere to it. What was particularly striking is that this electronic alteration was shown to have a far-reaching impact, allowing a chain-reaction effect of enhanced adsorption even at distances reaching several micrometers. Such a vast range of influence is revolutionary, suggesting that even minimal quantities of gold could amplify the photocatalytic efficiency significantly, addressing a major limitation in contemporary photocatalysis methods.
Beyond Wastewater Treatment: Broader Implications
The implications of this research extend far beyond advanced water treatment systems. Understanding the long-range effects of nanoparticles not only enhances photocatalysis for pollutant degradation but could also revolutionize fields such as environmental sensing and solar energy harvesting. By better leveraging the properties of metals within semiconductor platforms, scientific disciplines can innovate more effective methods to convert light into chemical energy and enhance sensor responsiveness, effectively creating a cross-disciplinary bridge that champions sustainability and efficiency.
The Future of Nanotechnology in Environmental Solutions
The integration of nanotechnology into environmental sciences provides a hopeful outlook in the ongoing fight against pollution. The findings of this Cornell University study indicate a forward path where traditional limitations in pollutant degradation technologies are challenged and overcome. As researchers continue to explore these breakthroughs, the potential for developing advanced materials that not only remove micropollutants but also repurpose waste into energy becomes more tangible. The era of addressing environmental challenges through innovative scientific solutions is upon us, with nanotechnology standing at the forefront. As we prioritize the health of our ecosystems, leveraging such discoveries will be crucial to building a sustainable future.

Leave a Reply