Recent advancements in the field of molecular and coordination chemistry signal a potential revolution in our understanding and application of oxidation mechanisms involving positive ions. Under the stewardship of Professor Ingo Krossing at the University of Freiburg, a dedicated team of researchers has achieved unprecedented results regarding the oxidation potentials of commonly utilized oxidizing agents such as Ag+ and NO+. Their study has resulted in a significant leap from traditional potentials of +0.65 / +1.0 V to remarkably higher values of +1.50 / +1.52 V. This transformation opens doors to innovative applications in areas such as electrocatalysis and advanced redox systems, suggesting a future where complex reactions become more feasible.
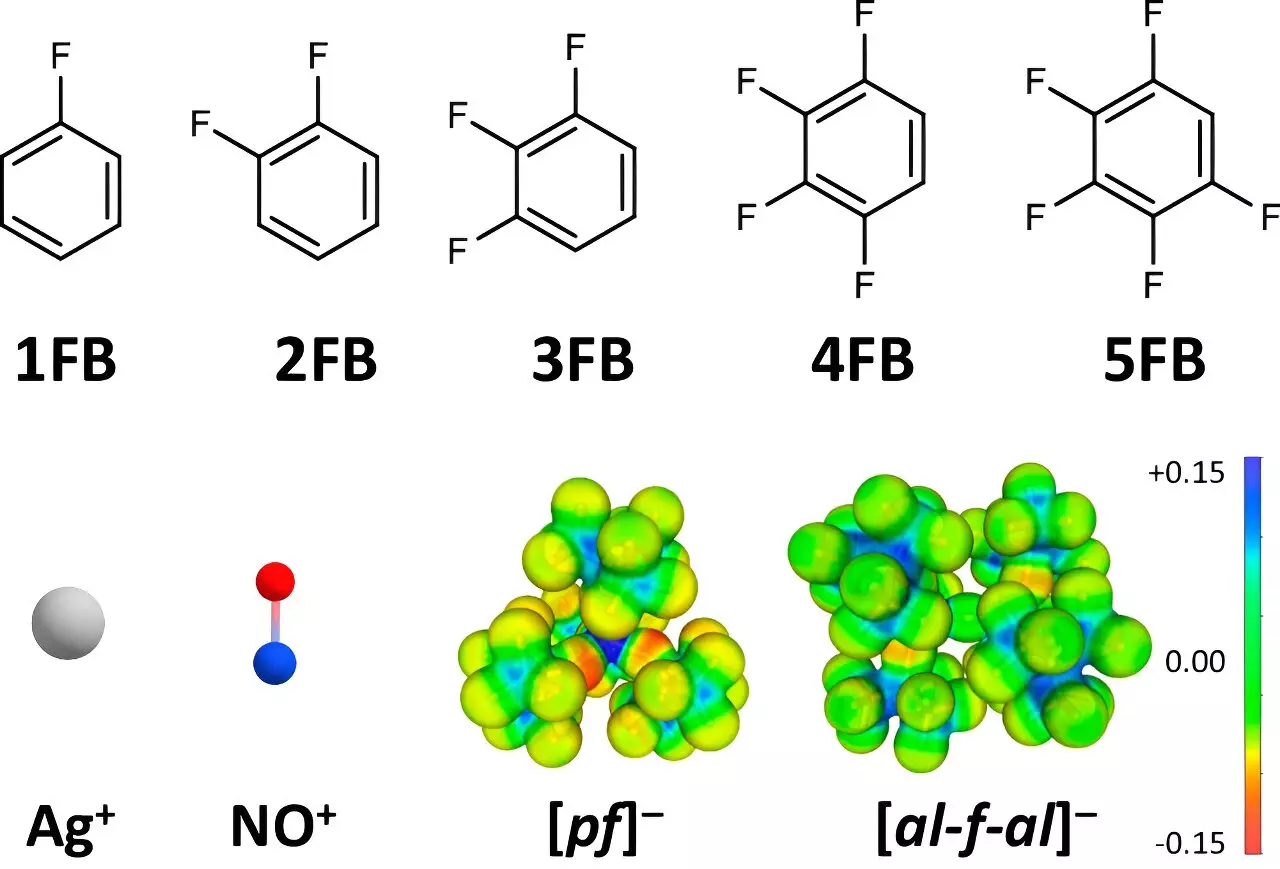
One of the central revelations of this research is the profound impact of the choice of solvents and anions on oxidation capabilities. Traditionally, researchers have relied on conventional solvents, which are often too interactive with the ions, leading to diminished oxidation potential. Krossing’s team tackled this challenge by employing weakly coordinating anions (WCAs) and strategically selected solvents, specifically fluorinated benzene derivatives. This decision was vital because it lessens the strong ion-environment interactions that typically restrict the capacity of positive ions to oxidize effectively.
The team meticulously analyzed various solvent properties, with Dr. Johannes Hunger from the Max Planck Institute for Polymer Research providing critical insights into dielectric constants. The findings unequivocally demonstrated that compounds with two to four fluorine atoms exhibited significant advantages over standard solvents such as dichloromethane and acetone. As the fluorination level increased, so did the ability of these solvents to maintain high ion oxidation potentials, effectively enhancing the ions’ reactivity.
Understanding the mechanics of ion interaction was crucial for this study. The researchers found that smaller ions with high charge density, like Ag+ and NO+, are typically subject to strong electrostatic forces with their environment. This interaction diminishes their oxidation potential, making reactions that involve these ions more challenging. Krossing and his team leveraged the unique properties of fluorinated solvents, detailing that as more fluorine atoms were added to the benzene derivatives, the interaction with ions like Ag+ and NO+ significantly reduced.
Dr. Malte Sellin, a co-author of the study, emphasized how employing single-crystal X-ray diffraction revealed that the solidity and stability of the compounds formed with the solvents were markedly improved. As the electrochemical measurements pointed to these nearly undisturbed particles, the researchers validated their hypothesis that enhanced fluorination leads to increased oxidation power.
The implications of Krossing’s work extend beyond mere interest in ion chemistry; it paves the way for extensive future research in various domains. The ability to carry out redox reactions with compounds that were previously considered difficult to oxidize has tantalizing potential, from advancements in energy storage systems to innovative applications in materials science and electrocatalysis.
Moreover, the presented findings could spearhead studies into the automation of chemical processes, streamlining the operational steps in industries reliant on these fundamental reactions. The combination of weak anion interactions and unique solvent properties may well give rise to methodologies that expedite chemical synthesis.
The groundbreaking research executed by Professor Ingo Krossing and his colleagues signifies a transformative shift in the landscape of molecular and coordination chemistry. By harnessing the potential of weakly interacting solvents and scrutinizing ion interactions at a granular level, they have unlocked new avenues for research and industrial application. As the scientific community begins to explore the myriad possibilities arising from this work, it is clear that we stand on the cusp of a new era of chemical exploration, promising a rich tapestry of innovations ahead.

Leave a Reply