Lung diseases are a significant global health crisis, accounting for millions of fatalities each year. Despite advancements in medicine, treatment options remain limited, presenting a formidable challenge for researchers and healthcare providers alike. Traditional animal models, primarily rodents, have been vital in developing treatments; however, they fall short in accurately mirroring the intricacies of human lung diseases like chronic obstructive pulmonary disease (COPD) and cystic fibrosis. Fortunately, recent developments in bioprinting technologies offer promising avenues for advancing our understanding and treatment of these debilitating conditions.
Currently, the management of chronic lung diseases often revolves around medications and, in severe cases, organ transplants. However, the demand for donor organs far exceeds supply, leaving many patients without viable alternatives. While pharmacological interventions can provide symptomatic relief, they do not address the core issues of these diseases, such as progressive lung tissue degeneration. The challenge lies in developing innovative treatments and models that can emulate human lung physiology and pathology more accurately.
Animal models have been the go-to solution for researchers aiming to better understand lung diseases. These models, though useful, have limitations; they may not provide reliable insights regarding human responses to experimental drugs or therapies. This mismatch underscores the urgent need for more human-relevant research models to advance therapeutic strategies for lung disease effectively.
Amid these challenges, scientists and bioengineers have turned to 3D bioprinting—an innovative technique that allows for the fabrication of complex tissue structures. By using bioinks, which serve as the “ink” in 3D printing processes, researchers can create biomimetic tissues that closely replicate the architecture and functionality of human organs. However, a significant hurdle has been developing bioinks that not only preserve the necessary cellular properties but also ensure proper nutrient diffusion and oxygenation within the printed construct.
Recent studies have shown promise in using mucin, a component of mucus known for its antibacterial properties, to create a novel bioink. Researchers led by Ashok Raichur have made significant strides by adapting mucin to enhance its suitability for bioprinting applications. By chemically modifying mucin into methacrylated mucin (MuMA) and integrating it with lung cells, they sought to produce a biocompatible material capable of supporting cell growth in a 3D printed environment.
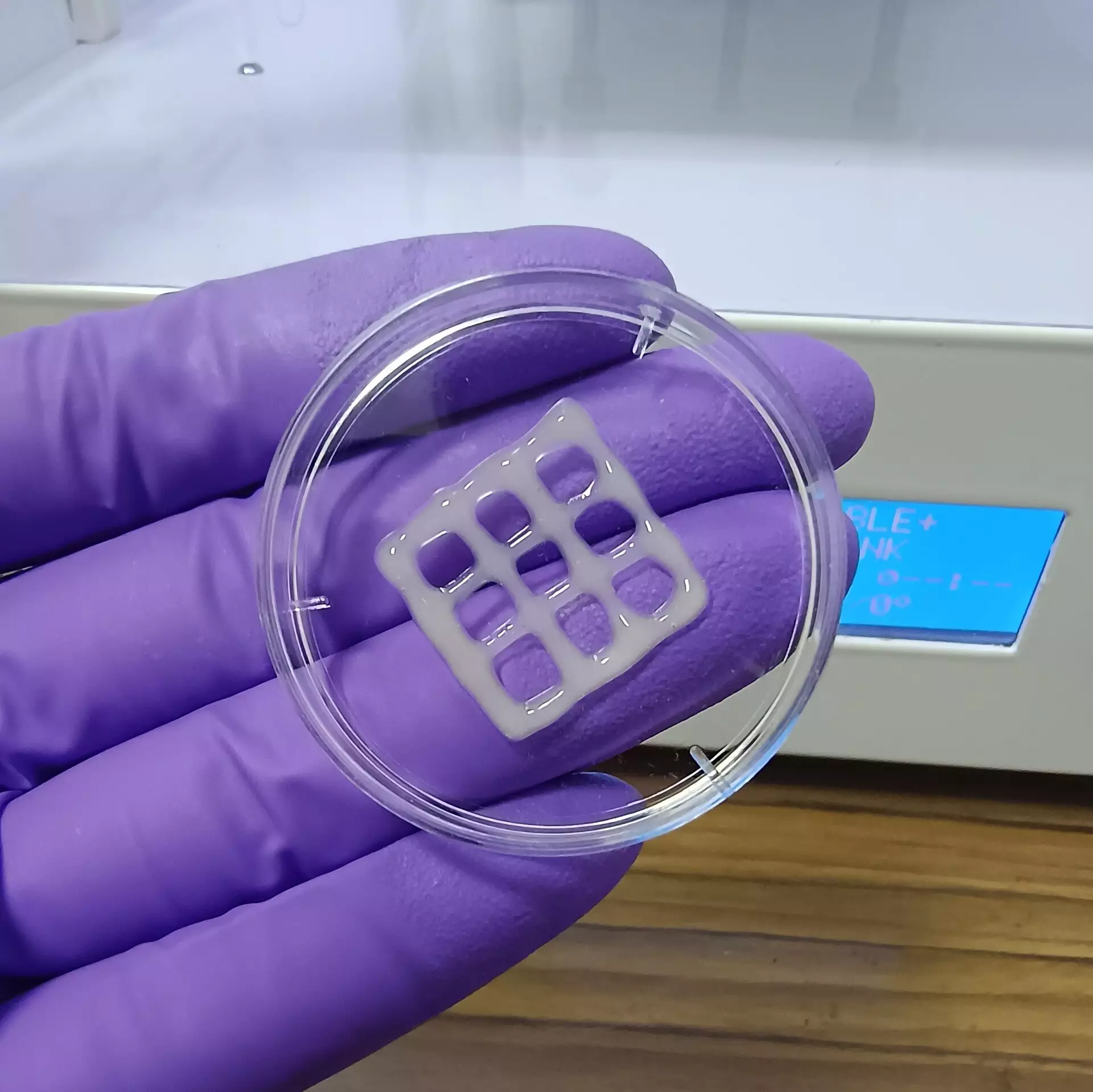
The team’s approach involved combining MuMA with hyaluronic acid, a naturally occurring polymer that enhances viscosity and promotes cellular adhesion. By mixing these components, they were able to produce a bioink that better supports lung cell growth while facilitating oxygen and nutrient exchange. After printing this bioink into specific patterns, exposure to blue light initiated crosslinking reactions that stabilized the printed structures. The resulting porous gel not only retained moisture but also provided an ideal microenvironment for lung cells to thrive.
Preliminary findings from this research indicate that these gel-like structures are non-toxic and possess bio-degradability under physiological conditions, which bodes well for future applications. Such implants could potentially be used in regenerative therapies where the scaffold would gradually dissolve as new lung tissue is formed. Moreover, the implications of this work extend beyond therapeutic uses; the bioink can also function as a sophisticated model for studying the mechanisms of lung diseases and testing novel treatments.
The development of this mucus-based bioink represents a significant leap forward in lung disease research. By providing a more accurate representation of human lung tissue, it paves the way for enhanced drug testing and evaluation of treatment efficacy. The capacity to fabricate lung tissue in vitro brings researchers closer to generating organ-on-a-chip models, revolutionizing preclinical testing and leading to safer, more effective treatments for patients suffering from chronic lung diseases.
As the field of bioprinting continues to evolve, the advent of novel bioinks such as those derived from mucin stands to transform the landscape of lung disease research. This innovation not only addresses the existing shortcomings of traditional animal models but also opens new avenues for therapeutic development and testing, potentially saving countless lives.


Leave a Reply