A groundbreaking study conducted by a team of researchers from Pohang University of Science and Technology (POSTECH), the Korea Research Institute of Chemical Technology, and Chonnam National University has led to the development of a revolutionary technique for separating well-mixed mixtures. Led by Professor Jee-hoon Han from the Department of Chemical Engineering at POSTECH, the research team has created a technology that enables the efficient synthesis and purification of ionic liquids. This cutting-edge research was recently highlighted as the cover paper in the online edition of Industrial & Engineering Chemistry Research.
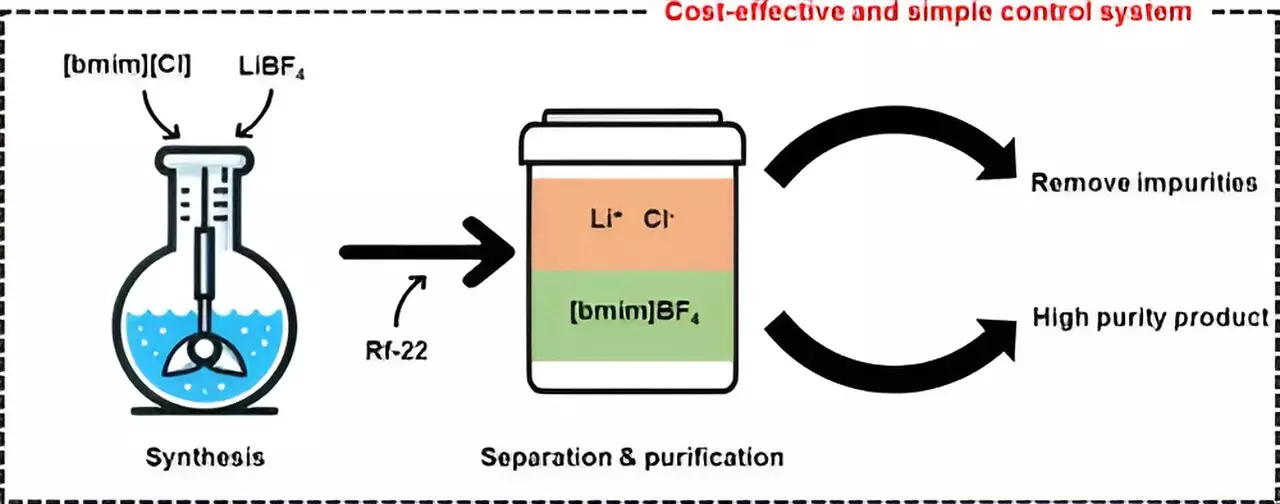
Ionic liquids are salts that possess the unique property of remaining in a liquid state at room temperature or even at relatively low temperatures due to strong electrical interactions between their ions. These liquids have gained popularity in various industrial applications such as catalysts and electrolytes due to their nonflammability, low volatility, and thermal and chemical stability. One of the most well-known ionic liquids, [bmim][BF4], is prized for its high stability and low toxicity. However, the cumbersome and costly process of removing impurities like lithium chloride (LiCl) during synthesis has impeded the commercialization of this technology.
In their study, the researchers utilized halocarbon refrigerants, specifically chlorodifluoromethane (Rf-22), to synthesize the ionic liquid [bmim][BF4] in a more cost-effective and efficient manner compared to traditional methods. By leveraging Rf-22 as a phase separation mediator, they were able to induce a mixture containing methylimidazole to separate into two distinct layers, similar to the separation of oil and water. Through experimentation with varying ratios of [bmim][BF4], water, and halocarbon mixtures, the team observed phase separation and utilized the data to create a ternary phase diagram model.
The Success of the Study
With the help of the ternary phase diagram modeling, the researchers achieved the production of high-purity [bmim][BF4] with a purity level exceeding 99%. Furthermore, they successfully recovered and recycled the layer containing methylimidazole that was not involved in the synthesis reaction. To evaluate the economic feasibility of their purification technology, the team conducted process simulations. Their cost analysis revealed that the minimum selling price for producing 1 ton of [bmim][BF4] per day would be approximately $12,000 per ton, making their method more competitive than existing technologies and showcasing its potential for commercialization.
The innovative technique developed by the research team offers a promising solution to the challenges associated with synthesizing and purifying ionic liquids. With its cost-effectiveness, efficiency, and ability to produce high-purity products, this technology has the potential to revolutionize the industrial applications of ionic liquids and pave the way for their widespread commercial use.


Leave a Reply