Recent advancements in the field of microbiology have shed light on the complex world of sphingolipids, specifically focusing on sphingomyelin and its implications in health and disease. A collaborative effort among researchers from Würzburg and Berlin has led to the introduction of a groundbreaking molecule designed to visualize the metabolism of sphingomyelin. This innovation is not only a significant step forward in fundamental research but also opens new avenues for therapeutic strategies, particularly in the context of infectious diseases. The study has been published in the prestigious journal Nature Communications.
Sphingolipids, which encompass a variety of lipid molecules, have their origins traced back to Ludwig Thudichum, a 19th-century German pathologist who first isolated these substances from the brain. Thudichum’s work, particularly his naming of sphingolipids after the enigmatic Sphinx, reflects the challenges these molecules present to researchers. Understanding and manipulating sphingolipid metabolism is a tantalizing challenge—one that holds the potential to unravel the mechanisms behind various diseases, including genetic conditions like Fabry’s disease and Gaucher’s disease as well as infectious diseases linked to bacteria and viruses.
Sphingomyelin is a key player in the spectrum of sphingolipids, known for its crucial role in cellular signaling and membrane integrity. In various infectious diseases, the enzyme sphingomyelinase facilitates the breakdown of sphingomyelin, which is often a vital step in pathogen invasion and replication. Historically, the inability to visualize this enzymatic activity has hindered advancements in infection research, leaving a gap in our understanding of these pathogenic processes.
The team from the Research Training Group 2581, encompassing chemists, physicists, and biologists, heralds a new chapter by creating a derivative of sphingomyelin that allows for the visualization of both the distribution of sphingomyelin and the activity of sphingomyelinase during infection. This trifunctional sphingomyelin is crafted to retain metabolic compatibility, a feat that has posed significant challenges to researchers in the development of similar molecules.
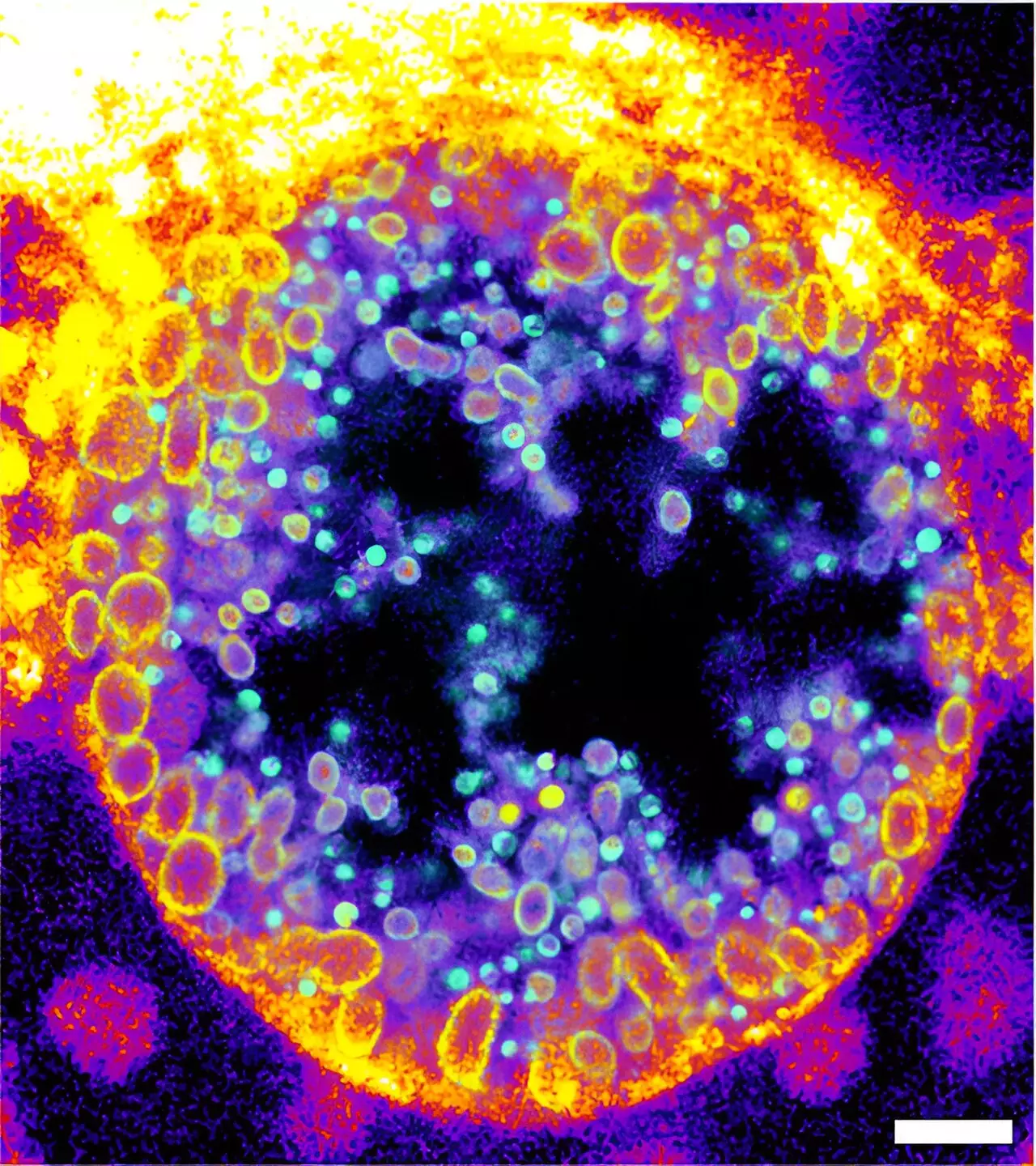
The innovation does not stop at molecular development; the researchers have implemented advanced visual techniques such as expansion microscopy and click-chemistry to provide insights into the dynamics of sphingomyelin degradation. In their experiments, they successfully observed the activity of bacterial sphingomyelinase on human cell surfaces and detailed the degradation process occurring within human cells during Chlamydia infections, a major concern due to its link to reproductive health and potential cancer development.
Chlamydia, known for creating a replicative organelle called an “inclusion,” serves as a perfect model for this research. By demonstrating that the inclusions primarily contain the byproducts of trifunctional sphingomyelin metabolism, the researchers reveal the critical connection between sphingomyelin degradation and the infectious cycle of the pathogen. The exploration of metabolized sphingomyelin molecules during the transition from non-infectious to infectious forms of Chlamydia highlights the molecule’s pivotal role in understanding infection mechanisms.
The implications of this research are vast. By enabling scientists to visualize sphingomyelin metabolism within the context of infections, new strategies for targeted therapies can be developed. This advancement not only contributes to a deeper understanding of existing infections but also paves the way for innovative approaches to combat emerging infectious threats.
Professor Jürgen Seibel from the Institute of Organic Chemistry at Julius-Maximilians-Universität Würzburg highlights the importance of these new chemical tools, asserting their potential for widespread application in laboratories globally. As researchers build on these findings, the hope is that a clearer understanding of sphingomyelin metabolism will lead to effective interventions against infections that currently lack adequate treatment options.
The recent developments in sphingomyelin visualization represent a critical advancement in infection research, reinforcing the profound connection between lipid metabolism and human health. As this field continues to expand, it may redefine approaches to both diagnosis and treatment in infectious disease, ultimately contributing to better patient outcomes.

Leave a Reply