In the ever-evolving field of chemistry, the quest for more efficient synthesis methods is continuous. Recently, researchers from the University of Illinois Urbana-Champaign have made significant strides toward this goal, focusing on the synthesis of ethers—essential components found in a myriad of products ranging from pharmaceuticals to personal care items. Spearheaded by Professor M. Christina White, the team published their groundbreaking findings in the prestigious journal Science, revealing a novel catalyst inspired by natural enzymatic processes. This innovation not only streamlines the synthesis of ethers but also allows the creation of previously elusive compounds.
Ethers play a pivotal role in the chemistry of various industries, serving as important functional groups in countless compounds. They are utilized in the formulation of drugs, food additives, personal care products, and many other consumer goods. However, traditional methods of ether synthesis often present challenges, including the need for extensive reaction protocols that can require large quantities of reactants and lead to complex mixtures of by-products. This highlights a critical need for improved synthetic pathways that save time, materials, and labor while producing the desired outcomes efficiently.
The conventional approach to synthesizing ethers typically involves activating an alcohol by removing a proton, thus making it reactive. While this textbook method does facilitate ether formation, it comes with significant drawbacks. The activation process can yield a confusing array of products that complicate purification, often resulting in lower yields of the desired product. Not to mention, the materials used are often in excess, contributing to inefficiencies. As graduate student Sven Kaster noted, finding an alternative method that does not rely on activating the alcohol nor utilizing large quantities of starting materials was essential.
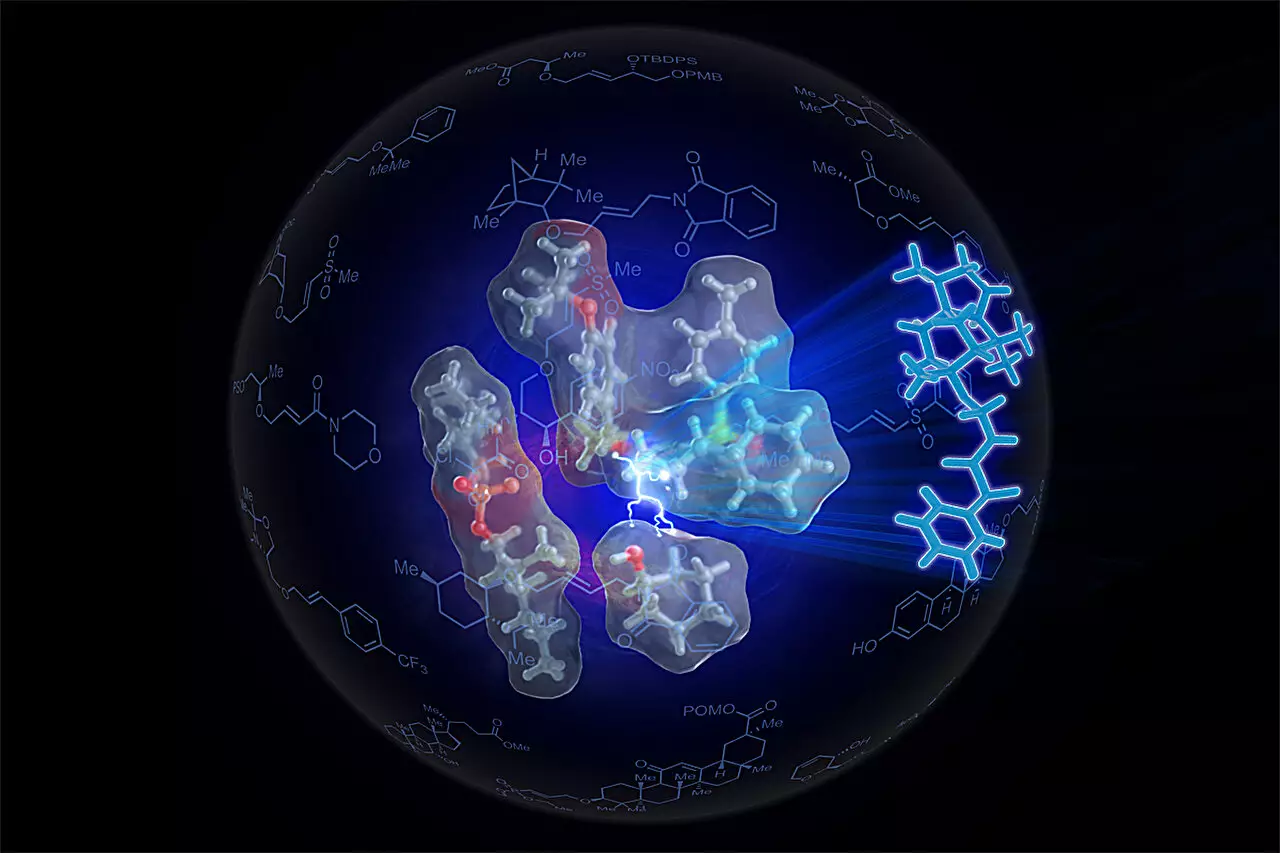
To address these challenges, the research team developed a groundbreaking self-assembling catalyst that incorporates palladium, which plays a crucial role in chemical transformations. Referred to as SOX, this catalyst actively facilitates the bond cleavage between carbon and hydrogen in an alkene, enabling it to react with an alcohol more efficiently. The innovative nature of this catalyst lies in its design, which ensures that the reactive partners are positioned and oriented correctly—akin to how natural enzymes operate in biological systems.
The significance of the SOX catalyst cannot be understated; it represents a paradigm shift in the way chemists approach ether synthesis. By aligning the reactants properly, the catalyst improves the likelihood of a successful reaction, thus paving the way for higher yields and reducing the complexity involved.
One of the most compelling aspects of this research is the inspiration drawn from the natural world. Just as enzymes in biological systems facilitate intricate reactions by bringing molecules together in ideal orientations, the Sven-SOX catalyst mimics this mechanism. By producing a tailored version of the catalyst with specific electronic and geometric properties, the researchers effectively allowed the activated alkene and alcohol to engage in a synergistic manner—much like two people reaching out to hold hands comfortably.
This biomimetic approach embodies a broader trend in chemistry, where scientists increasingly look to nature for innovative solutions to synthetic challenges. The efficiency and specificity achieved in this study underline the potential benefits of combining natural principles with modern synthetic chemistry.
The research team’s accomplishment is nothing short of remarkable. Utilizing the Sven-SOX catalyst, they successfully produced over 130 different ethers, including some that had previously remained elusive. This achievement signifies not only a breakthrough in methodology but also the potential for creating new compounds with unique functionalities. Such versatility opens up exciting opportunities for research and development across various sectors.
Additionally, the mild reaction conditions associated with the catalyst allow for the inclusion of sensitive functional groups that traditional methods would deem incompatible. This feature significantly broadens the scope of potential reactions, further solidifying the catalyst’s value.
Encouraged by their success, the researchers plan to delve into other classes of small-molecule catalysts that could embody enzyme-like characteristics, aiming to revolutionize the synthesis of an array of chemical classes. The implications of this research extend well beyond ethers; the principles learned here could inform future catalyst design in numerous chemical applications.
As Professor White aptly stated, this work emphasizes the importance of fundamental scientific research and the power of small molecules in achieving enzyme-like efficiency. By harnessing the tools of nature, chemists can explore new pathways for synthesis, ultimately propelling the field of chemistry into a promising future filled with potential innovations.
The work conducted by the University of Illinois Urbana-Champaign represents a significant leap forward in synthetic chemistry. By learning from and emulating nature’s mechanisms, researchers have developed efficient, innovative solutions to long-standing challenges in chemical synthesis, benefitting not only the scientific community but society as a whole.


Leave a Reply