Esters, the chemical compounds responsible for the sweet smell of fruits like strawberries, have a wide range of applications in various industries, from pharmaceuticals to cosmetics. However, the conventional methods used to break down esters to produce desirable alcohols and other chemicals can be costly and harmful to the environment. Scientists have traditionally relied on highly reactive and difficult to handle metal reductants for ester reduction, leading to financial and environmental consequences.
A team of researchers at the National Institutes of Natural Sciences (NINS) in Japan has developed a novel approach to ester reduction using light as an energy source. In a surprising twist, instead of removing electrons from esters to break them down, the researchers actually add electrons to the compounds. This addition of electrons causes the ester groups to reduce to more basic components.
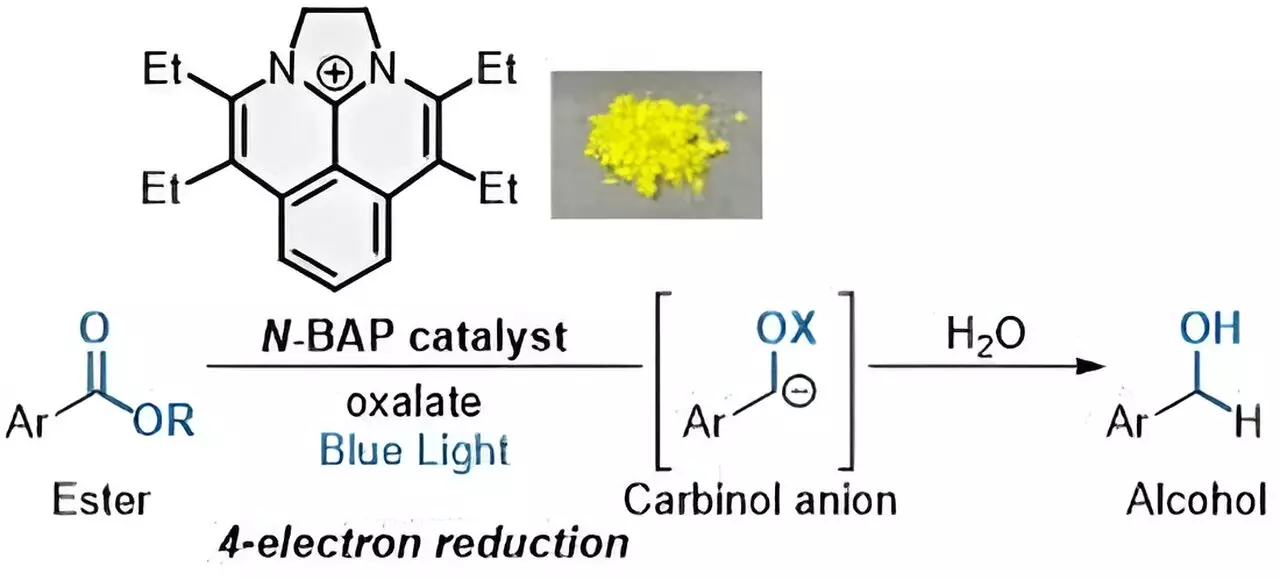
The researchers are exploring the use of sustainable photocatalysts in the ester reduction process. These catalysts, activated by light, facilitate electron transfer processes without the need for highly reactive metal reductants. Unlike traditional photocatalysts that use expensive noble metals and only add one electron to compounds, the newly developed photocatalyst “N-BAP” can add four electrons in quick succession, allowing for the efficient reduction of esters to form alcohols.
According to Shintaro Okumura, assistant professor at the Institute for Molecular Science (IMS) of NINS, photocatalytic reactions have gained attention as sustainable methods aligned with the United Nations’ Sustainable Development Goals. The ability to reduce esters to form alcohols through a quadruple electron transfer process presents a significant advancement in organic synthesis. The combination of the N-BAP photocatalyst with oxalate as a traceless reductant enables the rapid reduction of esters, paving the way for the production of valuable chemicals with minimal environmental impact.
The successful development of this novel photocatalytic approach to ester reduction opens up possibilities for more sustainable and cost-effective chemical synthesis processes. By harnessing the power of light to drive chemical reactions, researchers are moving towards a greener future in the field of organic chemistry. The ability to selectively manipulate chemical compounds through multi-electron transfer processes holds promise for the development of new pharmaceuticals, cosmetics, and other valuable products.
The groundbreaking research conducted by the team at NINS represents a significant milestone in the field of organic chemistry. By reimagining the process of ester reduction and leveraging the potential of sustainable photocatalysts, the researchers have laid the foundation for a more environmentally friendly approach to chemical synthesis. This innovation not only offers economic benefits but also contributes to the global effort towards achieving sustainable development goals.


Leave a Reply