The preservation of medical treatments, ranging from vaccines to blood donations, is a fundamental aspect of modern healthcare. The recent advancements made by researchers at the University of Warwick and the University of Manchester herald a new paradigm in the field of cryopreservation. Their innovative computational framework promises to enhance the freezing and storage techniques essential for a plethora of medical applications, ensuring that vital treatments remain effective until they are needed.
Cryoprotectants are substances utilized to prevent the damaging effects of ice crystal formation during the freezing process. Their importance cannot be overstated, particularly for sensitive biological materials that require careful handling to maintain their efficacy. Historically, the employment of these compounds has been marred by the lengthy, trial-and-error processes designers must engage in to identify suitable candidates. Until now, most approaches relied heavily on empirical experimentation, resulting in significant costs and prolonged timelines that hinder research and development.
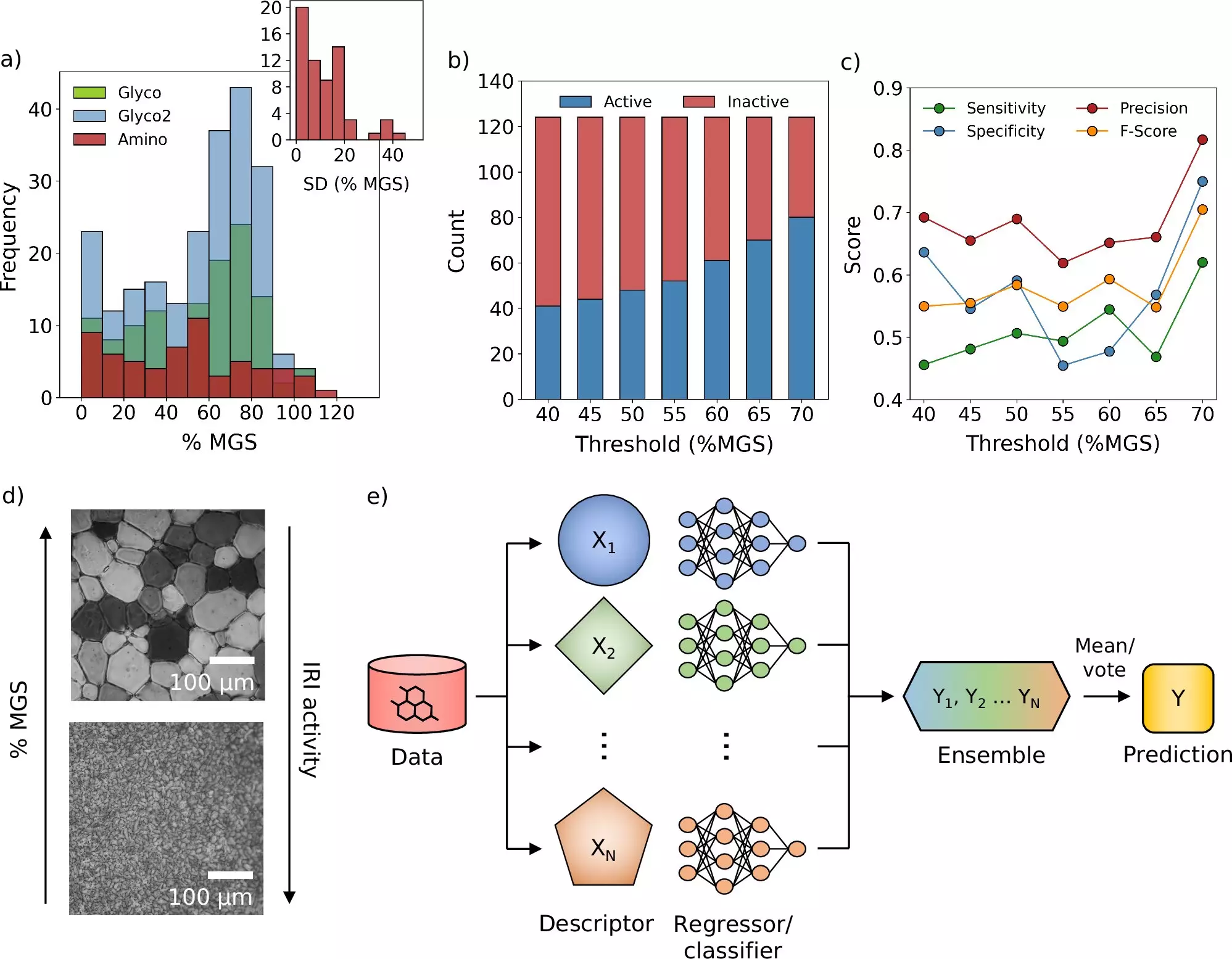
The breakthrough by the Warwick and Manchester teams seeks to address these inefficiencies. By merging a machine learning-based model with molecular simulation techniques, they have initiated a data-driven screening process that can assess hundreds of new potential cryoprotectants virtually. This not only expedites the discovery phase but also diversifies the pool of potential candidates, enhancing the possibilities for finding more effective solutions.
While machine learning is often hailed as a panacea in scientific domains, researchers caution against over-reliance on its capabilities. Professor Gabriele Sosso, who led this pioneering initiative, emphasized that machine learning is instrumental but not a catch-all remedy. Indeed, its integration with rigorous molecular simulations and experimental validation is what rendered this research successful.
The application of machine learning in this context is not merely an algorithmic exercise; it fundamentally shifts the methodological approach toward identifying cryoprotective agents. By exploring vast libraries of chemical compounds and eliminating ineffective candidates before engaging in costly laboratory tests, researchers can allocate their time and resources more efficiently. Dr. Matt Warren, who played a key role in this project, noted the excitement generated by transitioning from laborious data collection to a streamlined predictive model. The reduced experimental burden allows scientists to focus on complex inquiries that still necessitate human insight and creative problem-solving.
A significant breakthrough from this research is the identification of a new molecule capable of inhibiting the growth of ice crystals during freezing and thawing. This discovery addresses one of the most pressing challenges in cryopreservation—ice crystal formation—which can compromise cellular integrity and function. Notably, existing solutions have only been partially effective, protecting cells but failing to prevent ice from forming altogether.
The research team’s computer model revealed several new molecules with promising cryoprotective properties, even leading to a reduction in the conventional cryoprotectant needed when preserving blood samples. This advancement not only represents a leap in efficiency but may significantly accelerate processes like blood washing post-freezing, resulting in quicker transfusion times—a crucial factor in emergency medical situations.
The implications of this research extend beyond merely enhancing existing cryopreservation techniques. They also open the door for potentially repurposing established molecules known to possess ice-inhibitory properties. By optimizing and modifying these compounds, scientists can possibly create a new generation of both synthetic and natural cryoprotectants, leading to improved outcomes in various medical applications.
Furthermore, Professor Matthew Gibson’s insights, drawn from over a decade of studying ice-binding proteins found in polar fish, highlight the collaboration’s transformative nature. His comments reflect the synergy that emerges when computational capabilities and long-standing biological expertise converge—leading to discoveries that defy traditional expectations.
The work of the Warwick and Manchester teams is an outstanding example of how interdisciplinary collaboration can drive significant advancements in scientific research. This novel computational framework not only heralds a new era in cryopreservation but also sets the stage for future breakthroughs that will ultimately save lives and enhance the effectiveness of treatments worldwide.


Leave a Reply