In the realm of synthetic chemistry, the creation of organic molecules is a daily occurrence, pivotal for various industries including pharmaceuticals, agriculture, and materials science. Traditionally, these reactions occur in liquid environments that facilitate interactions between substrates and catalysts. However, the prevalent reliance on organic solvents presents a significant environmental and health dilemma. These solvents are not only toxic but also responsible for over 80% of the waste produced in chemical reactions, often leading to improper disposal and harmful ecological consequences. Synthetic chemists face the challenge of balancing reaction efficiency with environmental sustainability.
A Groundbreaking Innovation: Surfactants from Agricultural Waste
Addressing this pressing issue, a collaborative team from the Indian Institute of Science has developed an innovative solution by creating a bio-based surfactant derived from agricultural waste. Their research centers on the synthesis of CNSL-1000-M, a surfactant made from cashew nut shell liquid (CNSL). This byproduct arises from the cashew roasting process and is abundant in India, the world’s second-largest cashew producer. Pritesh Keshari, a Ph.D. student leading the study, emphasizes the importance of utilizing a bio-based replacement for organic solvents, aligning with both ecologically responsible practices and economic viability.
The Mechanism of Micellar Catalysis
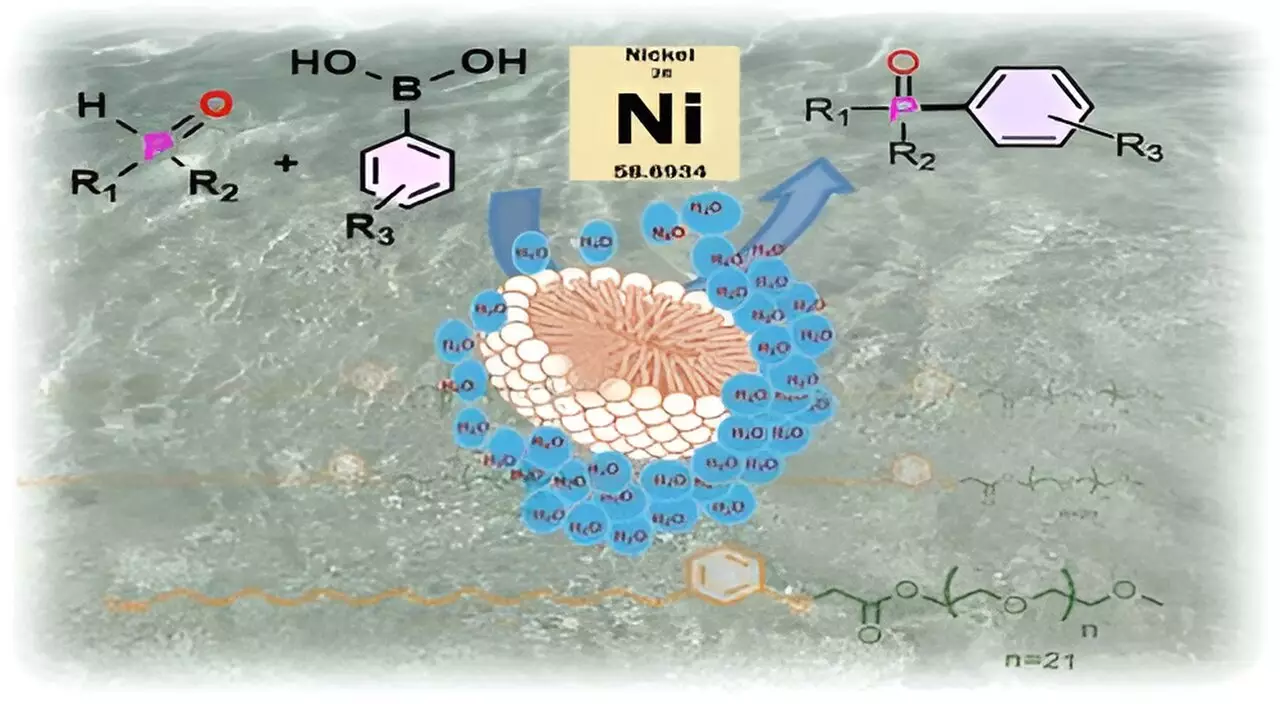
At the heart of this research lies micellar catalysis, a method that leverages the unique properties of surfactants to enable chemical reactions in water without sacrificing reaction efficiency. Surfactants exhibit dual characteristics—hydrophilic (water-attracting) and hydrophobic (water-repelling)—allowing them to self-assemble into micelles when dispersed in an aqueous environment. The researchers successfully combined cardanol, a hydrophobic component derived from CNSL, with m-PEG, a hydrophilic polymer. This combination forms micelles that create a protective, water-free environment conducive to reactions involving sensitive substrates and catalysts, such as nickel complexes.
The analogy used to describe this phenomenon is particularly illustrative; akin to a football floating on water, the micellar structure creates a distinct separation, enabling the chemistry to occur safely within the hydrophobic pocket, shielded from moisture. This ingenious solution not only mimics biological systems found in nature but also facilitates reactions essential for developing pharmaceuticals and high-tech materials.
The application of CNSL-1000-M in catalyzing the formation of crucial carbon-phosphorus bonds yielded remarkable results. Research revealed an impressive 80% increase in product yields when reactions were conducted in aqueous environments compared to conventional organic solvent methods. Not only did this surfactant surpass existing alternatives, providing a 30% boost in yields for reactions using water, but it also opened avenues for cost-effective practices by enabling the replacement of expensive catalysts, such as palladium, with cheaper nickel complexes. This shift not only reduces material costs but also aids in carrying out reactions at reduced temperatures, minimizing energy usage.
The potential implications of this research extend far beyond the laboratory. The researchers express a strong desire to collaborate with industries to facilitate the transition from toxic organic solvents to sustainable micellar technologies. Assistant Professor Susanta Hazra, a key figure in the study, envisions the application of this innovative approach at an industrial scale, highlighting the importance of further exploring micellar chemistry to maximize its benefits.
The Path Forward: Embracing Sustainability in Chemistry
In summation, the research conducted at the Indian Institute of Science paves the way for a paradigm shift in synthetic chemistry. By harnessing agricultural waste to create a sustainable surfactant for micellar catalysis, the team not only addresses the significant environmental issues caused by traditional organic solvents but also enhances reaction efficiency. The findings of this study hold promise for a greener future in chemical synthesis, encouraging industries to adopt more responsible practices that align with global sustainability goals. In a world increasingly focused on ecological conservation, this innovative approach presents a compelling case for the evolution of chemical processes toward a more sustainable and efficient framework.

Leave a Reply