The chemical landscape has been significantly impacted by findings that utilize dinitrogen (N2), a ubiquitous molecule in our atmosphere, as a primary reagent in synthesizing vital industrial compounds. Research conducted at RIKEN and published in the prestigious journal *Nature* introduces a groundbreaking method that could enhance the energy efficiency of alkyl amine synthesis. In an age where sustainable practices are more crucial than ever, this research paves the way for a more resourceful approach to chemical synthesis.
The Challenge of Dinitrogen Utilization
Dinitrogen, constituting nearly 80% of the air we breathe, is a remarkably stable molecule due to its strong triple bond. Historically, this stability has posed a significant hurdle for chemists wishing to incorporate dinitrogen into chemical reactions. The conventional approach for utilizing dinitrogen often necessitates its conversion into ammonia through the Haber-Bosch process, a method that, while somewhat effective, entails high energy requirements and is time-consuming. This method also relies on prior activation of alkenes, invariably complicating the synthesis pathway as it mandates additional steps that hamper efficiency.
“But why not use dinitrogen directly?” This question has lingered in the academic and industrial circles, perplexing researchers and prompting a search for innovative methodologies that can streamline dinitrogen’s application. The existing methods consume substantial energy and are not environmentally friendly, which accentuates the urgency for improvement in this domain.
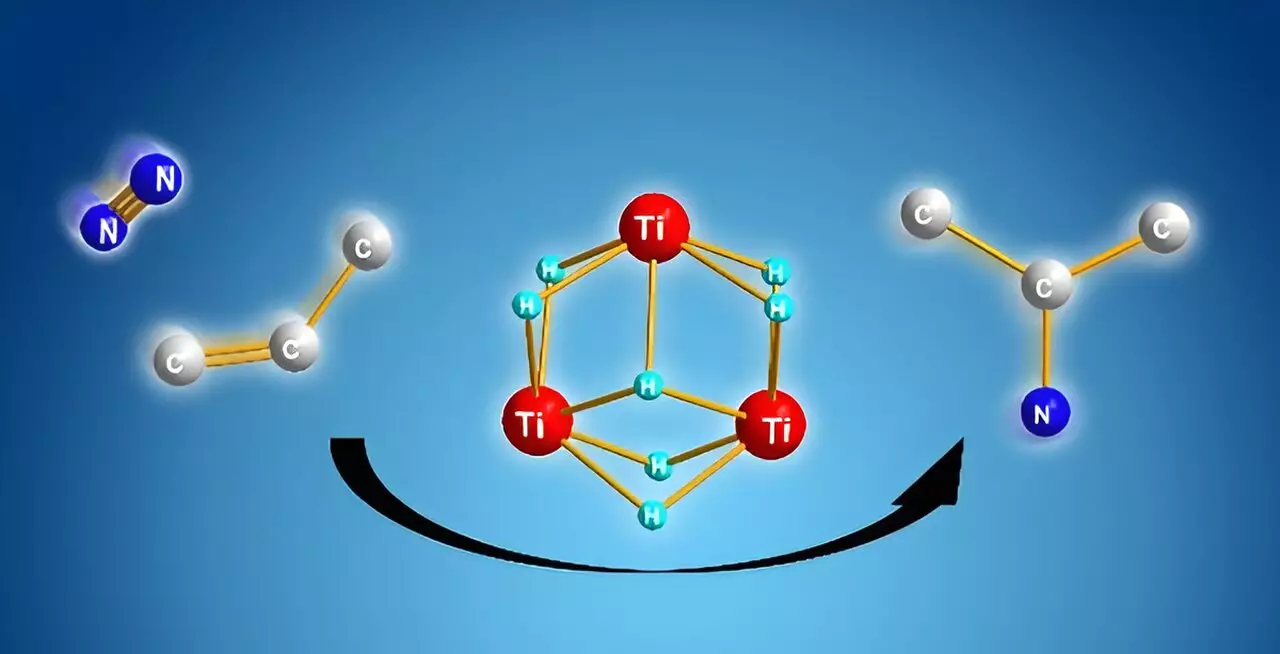
Fortunately, the research spearheaded by Takanori Shima and his colleagues from the RIKEN Center for Sustainable Resource Science presents an intriguing solution. Utilizing titanium polyhydrides—chemical complexes wherein titanium atoms are interconnected by hydrogen—showcased an unexpected reactivity toward stable molecules such as dinitrogen and benzene. This natural adaptability of titanium polyhydride demonstrates its potential as a groundbreaking catalyst in chemical synthesis.
The transformative aspect of Shima’s work lies in the cooperative behavior of multiple titanium–hydride units during reactions. Instead of the dinitrogen molecule being used indirectly through ammonia synthesis, this new method enables the direct cleavage of dinitrogen when combined with activated alkenes. The cooperative interaction among titanium–hydride units is crucial, as it provides a facilitated mechanism that allows for the successful formation of nitrogen-carbon bonds, yielding alkyl amines.
The key to understanding this innovative methodology lies in computational analysis performed by Shima’s team. It was revealed that after the activation of both the nitrogen and carbon components, the formation of nitrogen-carbon bonds occurs more favorably than alternative routes such as nitrogen-hydrogen or carbon-hydrogen bond formations. This selectivity is immensely advantageous, as it ensures efficient transformation without the propensity for less desirable byproducts that could complicate commercial applications.
A critical assessment of this work suggests that the energy efficiency afforded by using dinitrogen directly can catalyze a shift in industrial synthesis processes. The direct application of readily available dinitrogen not only reduces costs but also minimizes waste, aligning with the broader push towards sustainable chemistry practices.
Looking forward, Shima and his collaborators are zealously pursuing pathways to develop this innovative transformation into a catalytic process. If successful, this could herald a new era of chemical manufacturing where energy consumption is drastically reduced, and reliance on synthetic intermediates is minimized.
The pioneering work at RIKEN highlights how a deeper understanding of chemical bonding and reactivity can lead to significant advancements in synthetic methodologies. By transforming the way researchers and industries approach dinitrogen, this research not only promises efficient production of essential compounds like alkyl amines but also reinforces the significance of sustainability in chemical processes—a necessity in today’s ecologically-conscious world. The future of chemical synthesis looks optimistic, with dinitrogen now poised for a pivotal role in industrial applications.

Leave a Reply