Recent advancements in the electrochemical reduction of carbon dioxide (CO2) herald a new era in sustainable chemical manufacturing. A pivotal study published in Nature Energy reveals significant insights into how CO2 can be efficiently transformed into high-value chemicals, such as ethylene and ethanol. This research is crucial for deconstructing the traditional chemical production paradigm, enabling practices that align more closely with environmental stewardship. As society grapples with climate change and its associated challenges, understanding these processes will be vital for achieving carbon neutrality in the chemical industry.
The study, led by a team of renowned researchers including Dr. Arno Bergmann, Prof. Dr. Beatriz Roldán Cuenya, and Prof. Dr. Núria López, utilized advanced methodologies like in-situ surface-enhanced Raman spectroscopy (SERS) and density functional theory (DFT). This innovative approach allows for a meticulous investigation of copper (Cu) electrocatalysts at the molecular level. Traditionally, the mechanisms underlying CO2 reduction were poorly understood, which hampered progress in catalyst design. However, this study has effectively demystified critical intermediates involved in the conversion process, paving the way for tailored catalysts that can enhance productivity and selectivity.
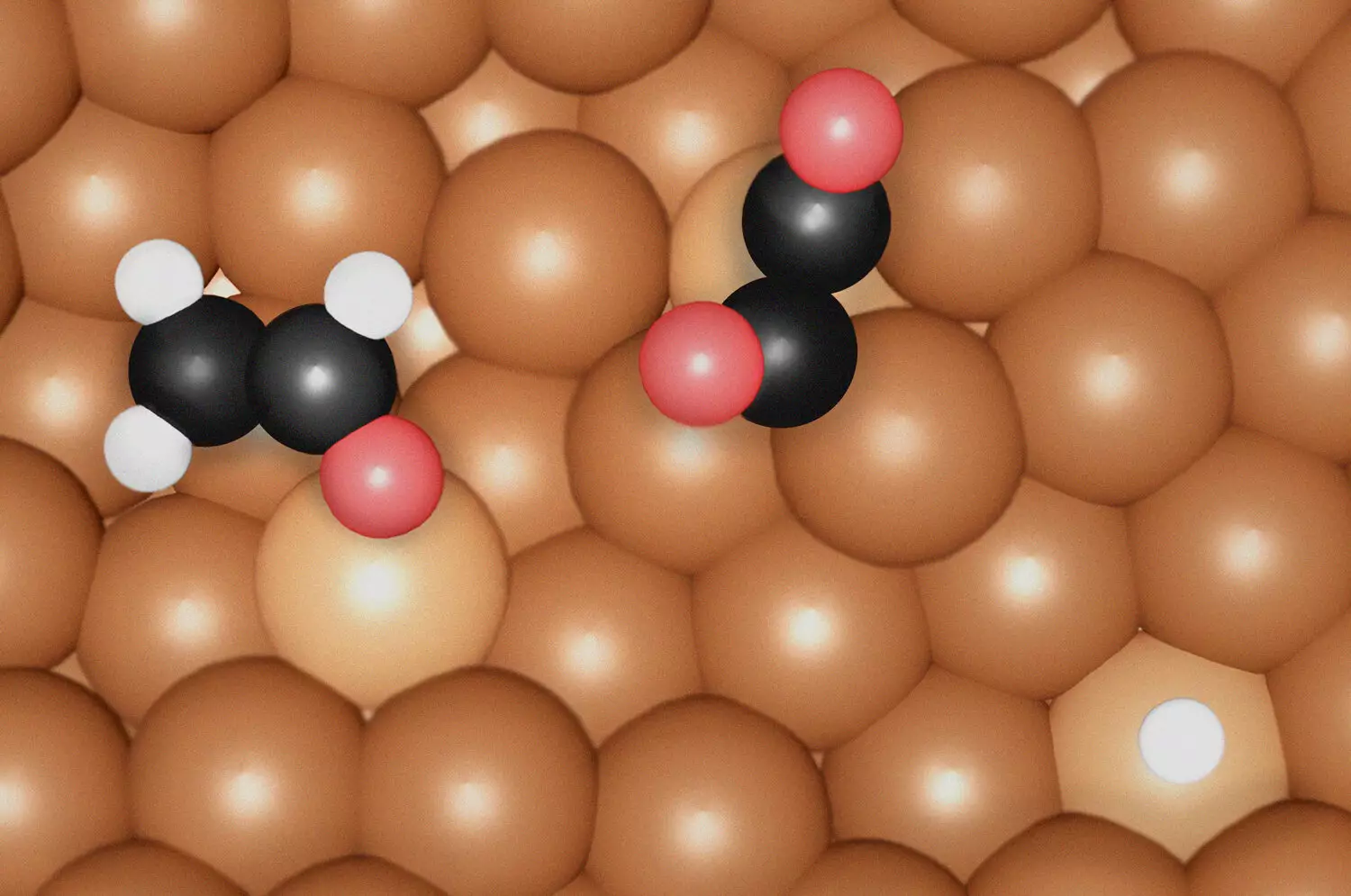
By focusing on the formation of *OC-CO(H) dimers at specific undercoordinated Cu sites, the research team elucidated the precise conditions under which ethylene is produced. In contrast, the formation of ethanol hinges upon the interplay between a highly compressed Cu environment and the intermediate *OCHCH2. This differentiated understanding allows researchers to strategically manipulate catalytic conditions, ultimately leading to increased efficiency in synthesizing these pivotal substances.
One of the noteworthy revelations from this study is the impact of surface morphology on catalysis. The undercoordinated Cu sites not only facilitate the bond formation of carbon monoxide (CO) — a critical component of the reduction process — but also display atomic irregularities that enhance the catalytic surface’s effectiveness. This finding suggests that certain conditions during the reaction can foster the emergence of these advantageous sites, indicating that the production environment itself plays a critical role in optimizing performance.
This depth of understanding highlights the need for ongoing research in optimizing catalysts at the atomic level. The interplay between structure and reactivity underscores the complex nature of catalysis and offers an avenue for further exploration in the quest for sustainable materials.
The findings reported in this study bear significant implications for the chemical industry. The conversion of CO2 into value-added products like ethylene — a precursor for eco-friendly plastics — and ethanol, which is vital for renewable fuel production, represent a promising solution to help mitigate greenhouse gas emissions. As industries seek to lower their carbon footprints, the ability to effectively utilize CO2 not only addresses environmental concerns but also fosters an innovative approach to raw material sourcing.
Moreover, by identifying the specific intermediates and optimal catalyst conditions necessary for selective production, this research lays the groundwork for designing advanced catalytic systems. Such innovations could revolutionize processes by making them more cost-effective and environmentally friendly, leading to a reduced reliance on fossil fuels while meeting global chemical demands.
This groundbreaking work is not a solitary achievement but rather a product of collaborative efforts between various research institutions, including the Fritz Haber Institute and the Institute of Chemical Research of Catalonia. By merging experimental findings with theoretical frameworks, the research team was able to construct a comprehensive understanding of CO2 reduction mechanisms.
Looking ahead, the focus must now shift toward translating these findings into practical applications. Continuous research and development, coupled with cross-disciplinary collaboration, will be essential in refining catalysis techniques and scaling them for industrial use. As scientists delve deeper into the intricate aspects of CO2 transformation, the potential for sustainable practices within the chemical sector remains promising.
This study signifies a monumental stride toward integrating CO2 reduction technology into mainstream chemical manufacturing. By unveiling the complexities of the conversion process and optimizing catalytic systems, we edge closer to a future where environmental sustainability is not just an aspiration but a reality.


Leave a Reply