In a groundbreaking study led by scientists from King’s College London in collaboration with Imperial College London, the active site of Acetyl-CoA Synthase, an enzyme crucial for capturing carbon from the atmosphere, has been successfully recreated. This research not only deepens our understanding of this important enzyme but also presents a promising new solution for carbon capture to combat climate change.
Acetyl-CoA Synthase (ACS) plays a vital role in the transformation of carbon dioxide into acetyl coenzyme-A, an essential molecule used in living organisms. This enzyme is most notably recognized for its involvement in the acetic acid cycle or Krebs Cycle, where acetic acid is oxidized to generate energy. Additionally, ACS is essential for both storing and releasing energy, as well as capturing carbon dioxide from the atmosphere and converting it into a more usable form.
Enzymes are intricate proteins that serve as biological catalysts, speeding up chemical reactions in living organisms. The complex chemical pathways facilitated by enzymes have evolved over billions of years and are incredibly challenging to study and replicate in laboratory settings. To better understand enzymes, scientists often create molecular models of the ‘active site’ to simulate the chemical reactions that take place.
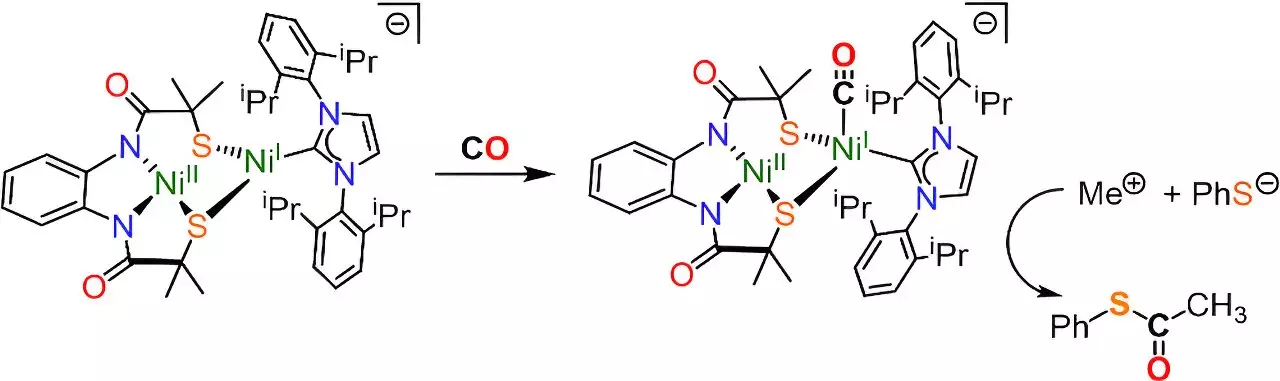
Despite previous attempts to replicate the active site of ACS in the lab, researchers faced challenges in accurately mimicking the shape and electronic environment necessary for capturing carbon. However, in this study, the team was able to create a molecular cluster featuring two nickel atoms that closely resembled the ACS enzyme’s active site. This model successfully replicated the chemical reaction of capturing atmospheric carbon and converting it into acetyl coenzyme-A.
The successful synthesis of acetyl coenzyme-A in the lab opens up new possibilities for better understanding the mechanism behind this crucial reaction. By employing advanced techniques such as Electron Paramagnetic Resonance spectroscopy, researchers can delve into the individual steps of the process and use this knowledge to design man-made catalysts for industrial applications. This breakthrough has the potential to revolutionize carbon capture technologies and pave the way for utilizing captured carbon in the production of biofuels and pharmaceuticals.
Looking ahead, the researchers hope that their novel model of the ACS enzyme’s active site will serve as a valuable tool for scientists working in enzyme spectroscopy. By sharing their findings and methodology, the team anticipates that other researchers can adapt and incorporate this model into their own studies of enzyme mechanisms. The inherent efficiency and speed of enzymes in nature pose a challenge for laboratory reproduction, but this breakthrough brings us one step closer to unlocking the full potential of these biological catalysts.
The recreation of the active site of Acetyl-CoA Synthase represents a significant milestone in enzyme research and carbon capture technology. With the ability to replicate this essential chemical reaction in the lab, scientists are poised to make strides towards developing sustainable solutions for combating climate change and utilizing captured carbon for various applications. This breakthrough opens up a world of possibilities for harnessing the power of enzymes in nature to address pressing environmental challenges.

Leave a Reply