In a groundbreaking endeavor, a dedicated team of researchers has made significant strides toward solving a major challenge in the realm of hydrogen production. Led by Professor Jeong Woo Han from Seoul National University, this robust group—including experts from Pohang University of Science and Technology (POSTECH) such as Professor Yong-Tae Kim, Dr. Sang-Mun Jung, and MSc student Yoona Kim—has developed an innovative catalyst for the hydrogen evolution reaction (HER). Their findings, which graced the cover of the journal Advanced Functional Materials, are not just an academic success; they hold the potential to transform the efficiency of renewable energy systems and contribute to a sustainable future.
The Importance of Hydrogen in Renewable Energy
As we venture deeper into the age of renewable energy, the variability of sources like solar, wind, and hydro power presents a notable challenge. Weather conditions play a dynamic role in energy generation, necessitating reliable methods for energy storage and delivery. This is where hydrogen emerges as a hero. Its ability to serve as a storage medium when coupled with water electrolysis systems offers a pragmatic pathway to harness renewable energy. The alkaline water electrolysis (AWE) system, in particular, has garnered attention for its cost-effectiveness and durability over alternatives. However, it is not without its complications.
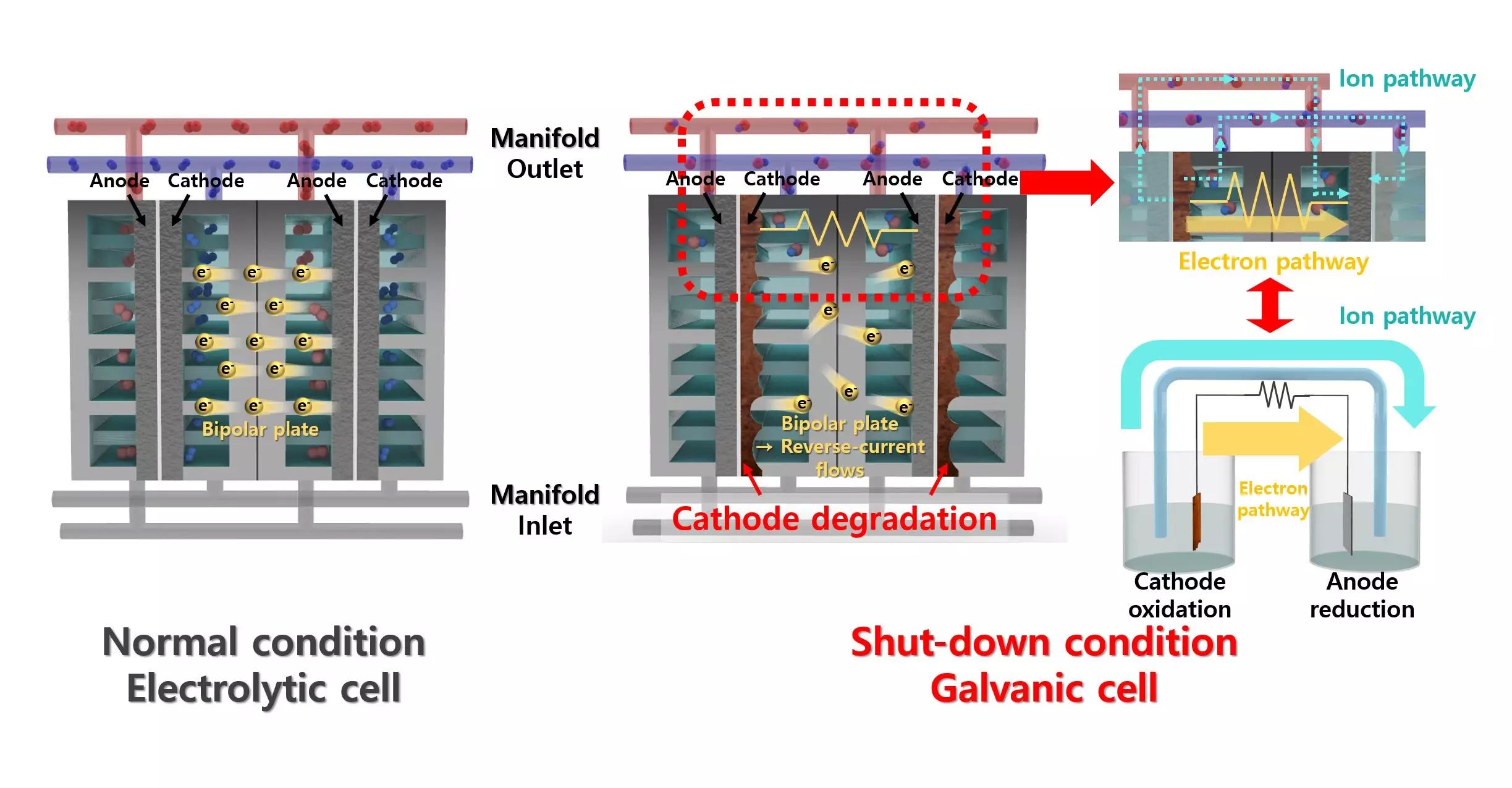
Addressing the Reverse Current Dilemma
The AWE system faces a formidable threat from reverse currents—temporary currents that can arise when the energy supply halts, leading to degradation of the vital electrodes. This degradation presents a significant barrier to harnessing the full potential of hydrogen production. The research team has brilliantly countered this challenge by introducing an unexpected innovation: a lead (Pb) coating on a nickel (Ni) catalyst. While lead is typically dismissed in catalysis due to its lackluster performance in HER, this study has brilliantly highlighted its potential when applied as a co-catalyst.
Co-Catalyst Breakthrough: Redefining Efficiency
The inclusion of lead isn’t merely a whimsical addition; it fundamentally revolutionizes how the nickel catalyst behaves under operational stresses. By promoting crucial processes like proton desorption and water dissociation, the lead coating enhances the catalyst’s efficiency markedly. This finding is especially poignant because it offers a dual advantage: not only does the modified catalyst improve the efficiency of hydrogen production, but it also enhances resilience against reverse currents—effectively shielding the electrolyzer from the dangers of degradation.
A Paradigm Shift in Catalyst Design
What sets this research apart is its practicality. Unlike previous approaches that demanded extra equipment to mitigate the effects of reverse currents, this advanced catalyst requires simply a thin layer of lead. This simplicity could urge industries toward the systemic adoption of this innovative solution without the burden of complicated and costly modifications. Professor Yong-Tae Kim has aptly remarked on the significance of their findings, indicating that this research represents a pioneering approach to address the reverse current challenges in AWE systems with a material-based strategy.
The implications of this research could be profound, dramatically impacting hydrogen production and storage in a world increasingly reliant on renewable energies. As the globe shifts toward sustainable practices, advancements such as these will be essential for supporting the transition and ensuring energy security.

Leave a Reply