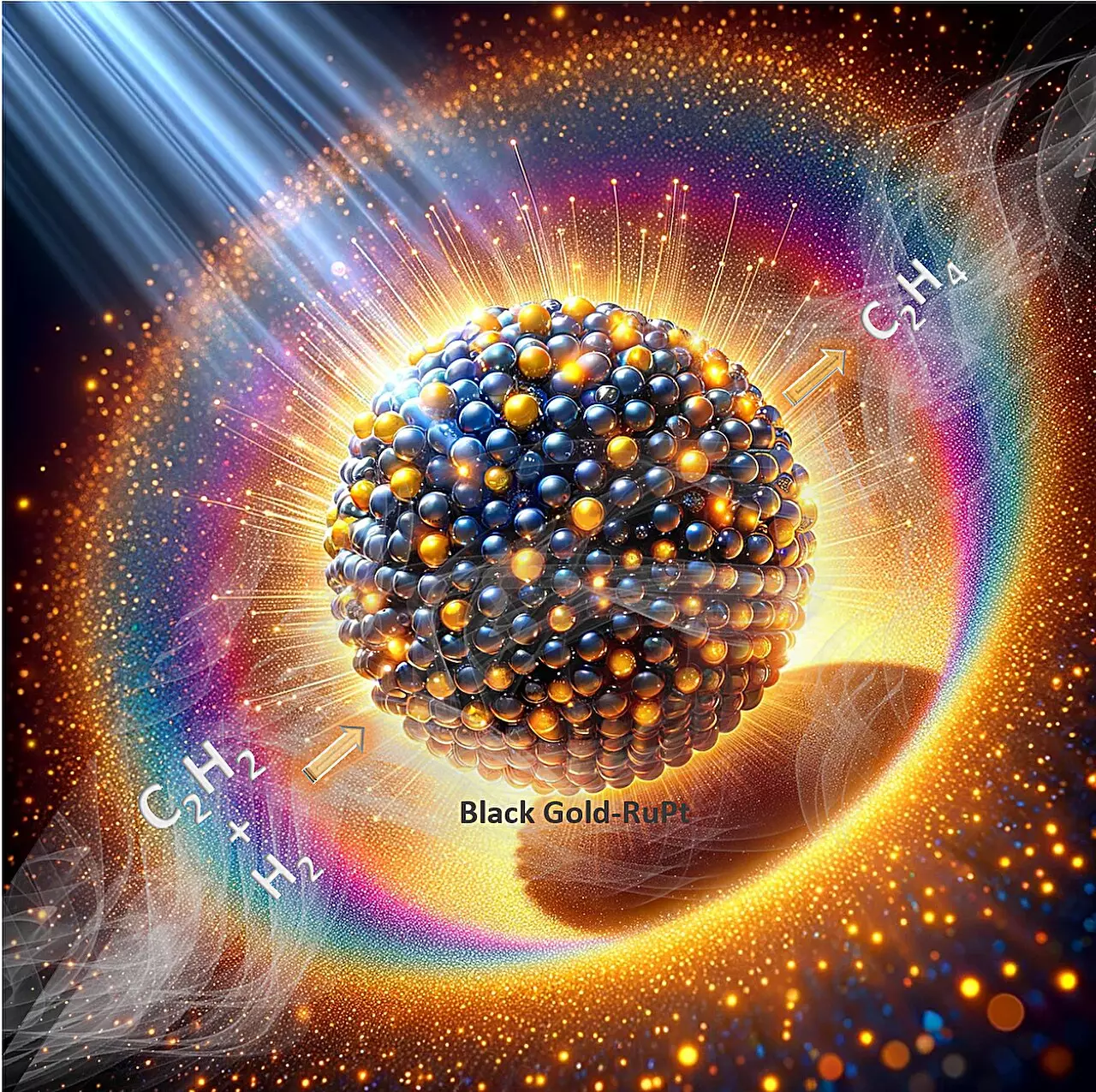
Researchers at Tata Institute of Fundamental Research (TIFR) in Mumbai have made a groundbreaking discovery in the field of catalyst development. Led by Prof. Polshettiwar, the team has successfully created a “plasmonic reduction catalyst stable in air” that surpasses the limitations of traditional reduction catalysts. This innovative catalyst combines platinum-doped ruthenium clusters and “plasmonic black gold,” allowing it to efficiently harvest visible light and generate hot spots that enhance its catalytic performance. The team’s findings were published in the journal Nature Communications.
Unlike traditional catalysts, this new catalyst exhibits remarkable performance in the semi-hydrogenation of acetylene, a crucial industrial process. Even with only visible light illumination and without external heating, the catalyst achieved an ethene production rate of 320 mmol g−1 h−1 with a selectivity of about 90%. This level of efficiency outperforms all previously known plasmonic and thermal catalysts. Interestingly, the catalyst’s optimal performance is only achieved when air is introduced during the reaction. Surprisingly, this unique requirement also contributes to the catalyst’s unprecedented stability for at least 100 hours. The researchers attribute this stability to plasmon-mediated simultaneous reduction and oxidation processes at the active sites during the reaction.
In order to gain a better understanding of the catalyst’s properties, the researchers employed finite-difference time-domain (FDTD) simulations. These simulations revealed a significant five-fold increase in the electric field, compared to the catalyst’s original state. This increase is due to the near-field coupling between the ruthenium-platinum nanoparticles and the catalyst, which plays a crucial role in activating chemical bonds. Furthermore, the catalyst’s effectiveness is evident in its kinetic isotope effect (KIE), which is larger under light illumination compared to darkness at all temperatures. This suggests that non-thermal effects play a significant role alongside photothermal activation of the reactants.
To gain insights into the reaction mechanism, the researchers conducted in-depth in-situ DRIFTS (Diffuse Reflectance Infrared Fourier Transform Spectroscopy) and DFT (Density Functional Theory) studies. These studies provided valuable information about the role of intermediates in selectivity, particularly on the oxide surface. The partially oxidized RuPt catalyst surface produces di-σ-bonded acetylene, which then undergoes several steps to ultimately yield ethene. These findings contribute significantly to our understanding of plasmonic catalysis and have the potential to revolutionize the development of sustainable and energy-efficient catalytic systems.
The development of this groundbreaking catalyst represents a tremendous leap forward in the field of catalysis. By defying the common instability of reduction catalysts in the presence of air, this catalyst offers enhanced performance and stability in the semi-hydrogenation of acetylene. Its ability to efficiently harvest visible light and utilize plasmonic coupling sets it apart from traditional thermal catalysts. The insights gained through various analytical techniques shed light on the mechanism behind the catalyst’s exceptional performance. This knowledge paves the way for the development of new, sustainable, and energy-efficient catalytic systems. The findings of Prof. Polshettiwar’s group at the Tata Institute of Fundamental Research open up exciting possibilities for future research and innovation in the field of plasmonic catalysis.


Leave a Reply