Recent advancements in quantum biology present a transformative opportunity to comprehend the enigmatic mechanisms behind Alzheimer’s disease. Traditionally, amyloid fibrils—protein aggregates located in the brains of individuals afflicted by Alzheimer’s—have been deemed detrimental. However, emerging research suggests that these fibrils might play a protective role against oxidative stress rather than merely acting as a causative factor in dementia. This insight challenges established notions about Alzheimer’s and calls for a reevaluation of treatment strategies directed at amyloid-related therapies.
Amyloid fibrils have long been the focal point in the Alzheimer’s research landscape, largely due to their presence in the brains of individuals with dementia. Standard treatment approaches have primarily aimed at lowering the levels of these fibrils, operating on the assumption that their reduction would lead to decreased cognitive decline. Yet, a perplexing observation has been made: a considerable number of individuals who exhibit significant amyloid deposition do not develop Alzheimer’s symptoms. This disconnect emphasizes the need to deeply analyze whether amyloids are truly the culprits or if they serve a more complex role.
In the past, scientists established a correlation between allostatic load—a term for chronic physiological stress—and dementia risk. While oxidative stress is known to contribute to the overall allostatic load, the relationship between this function and amyloid accumulation warrants closer inspection. Instead of solely focusing on destroying amyloid fibrils, alternative interpretations must be considered.
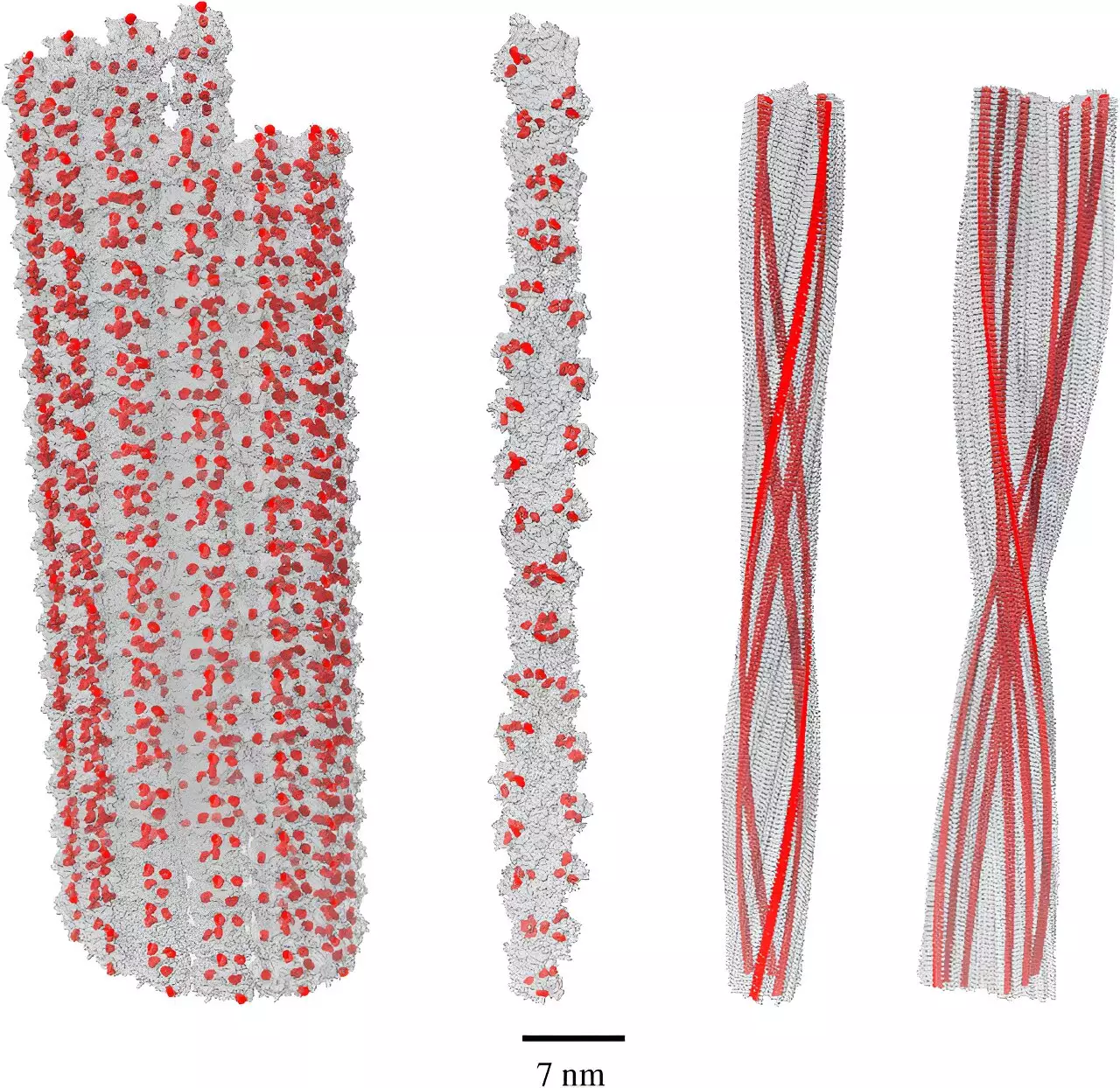
Dr. Philip Kurian and his research team from the Quantum Biology Laboratory at Howard University have pioneered investigations into the concept of single-photon superradiance, a phenomenon wherein molecules within a collective structure can collectively emit light. Their earlier findings indicated that tryptophan, an amino acid prevalent in many proteins, possesses the ability to withstand the body’s chaotic environment, retaining its capacity to efficiently handle oxidative stress.
Recent studies expanded this concept, confirming that amyloid fibrils, which are rich in tryptophan, manifest an even greater potential for superradiance than previously observed protein networks. This suggests that amyloid structures can absorb high-energy photons, synthesized during oxidative stress, and convert them to a safer energy level, providing a protective mechanism against cellular damage.
The implications of these findings underscore a significant paradigm shift. Rather than viewing amyloid fibrils as mere pathological agents in Alzheimer’s, Kurian’s research posits that these structures could serve as a biological response to environmental stressors, offering protective benefits against oxidative damage. If confirmed, this theory not only reshapes our understanding of Alzheimer’s disease but also proposes that amyloids might be an adaptive feature in the landscape of neurodegeneration rather than a destructive force.
The conversation surrounding amyloids has historically been one-dimensional, yet Kurian’s work invites scientists to think about the dynamic interplay between amyloids and the cellular environment. In many ways, these aggregates might be functioning as biological shields, rather than the villains of Alzheimer’s pathology.
Towards a New Paradigm of Alzheimer’s Research
Moving forward, researchers are called upon to broaden their lenses when considering treatment modalities for Alzheimer’s disease. The suggestion that amyloid fibrils could provide photoprotection rather than harm offers a fresh avenue for therapeutic exploration. If amyloids prove to be an evolved response to cellular stress, then the quest for Alzheimer’s therapies may begin to pivot from eliminating amyloids to leveraging their innate protective qualities.
Kurian anticipates that this research will reach across disciplines, connecting quantum mechanics with biological sciences in unprecedented ways. Recognizing the implications of light interactions and quantum phenomena might not only redefine Alzheimer’s disease paradigms but also enhance our understanding of broader biological processes, ultimately affecting how we approach diseases characterized by neurodegeneration.
The intersection of quantum biology and neurobiology offers a revolution for our understanding of Alzheimer’s disease. The groundbreaking findings regarding the role of amyloid fibrils challenge long-held assumptions about their function and potential harm. By embracing an interdisciplinary approach, researchers can explore these novel insights, unraveling new treatment possibilities that prioritize the complexity of biological responses to stress. As Kurian noted, the significance of light and quantum matter interactions in living systems is profound and warrants exploration beyond traditional scientific boundaries. The future of Alzheimer’s research may hinge on this new understanding, fostering hope in the pursuit of effective cures.

Leave a Reply