Chemistry researchers at Flinders University have discovered a new way to produce ‘green’ polymers using low-cost building blocks and a small amount of electricity. The reaction occurs quickly and at room temperature, without the need for hazardous chemical initiators. The research team has published an article in the Journal of the American Chemical Society detailing the potential uses of this method, including gold mining and recycling e-waste.
Details
The production of hundreds of millions of tons of plastic every year, with up to half of it being used for single purposes, has led the Flinders University research group to pursue more sustainable options. The use of electricity to create new materials is an emerging field of research that opens many doors to new chemicals and polymers that can be produced in a more sustainable way, according to co-author Dr. Thomas Nicholls, an expert in using electrochemistry to make valuable molecules.
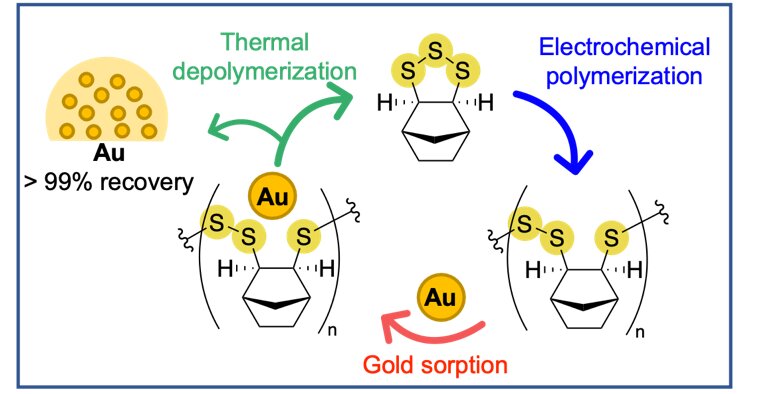
The process begins with the addition of an electron to the basic building block or monomer. The monomer is then ‘electrocuted’, and it reacts with another building block in a chain reaction, leading to the formation of a polymer. The power used in production has been identified as a contributor to pollution and global warming, making this new method of producing green polymers a sustainable alternative.
The key polymer produced by the team has sulfur-sulfur bonds in its backbone, which allows it to bind precious metals such as gold. The team has demonstrated that the key polymer can remove up to 97% of gold from solutions relevant to mining and e-waste recycling. The sulfur-sulfur bonds are also capable of being broken and reformed, which has enabled the team to discover conditions to convert the polymer back to its original building block. This is an important advance in recycling, as it allows the building block to be used again to make new polymers.
Typically, common plastics are recycled by heating and reshaping them into a new product. This process can cause degradation and down-cycling, leading to eventual disposal in landfill. In contrast, the polymers produced by the Flinders University scientists can be chemically converted back into their constituent building blocks in high yield, making it possible to create new polymers from the original building blocks.
The team also carried out quantum mechanical calculations to understand the details of how the reaction works. They discovered that the polymerization has a self-correcting mechanism that reverses whenever the wrong reaction occurs, ensuring a uniform polymer. This is an interesting and fortuitous finding that highlights the potential of this new method of producing green polymers.
Future applications of this class of materials include environmental remediation, gold mining, and the use of the polymer as an anti-microbial agent. The use of electricity to create new materials is expanding rapidly due to its versatility, and it may generate less waste than traditional chemical syntheses. Additionally, it can be powered with renewable energy, making it a sustainable alternative to existing methods.
The new method of producing green polymers discovered by the Flinders University research team is an exciting development in the field of electrochemistry. The method offers a sustainable alternative to existing methods of producing plastic, and it has the potential to be used in a range of applications, including environmental remediation, gold mining, and the creation of anti-microbial agents. The team’s findings also highlight the importance of pursuing sustainable alternatives to existing methods of production.


Leave a Reply