The activation of G protein-coupled receptors (GPCRs) is responsible for relaying molecular signals in the body, which affects various bodily processes. It is estimated that around one-third of existing drugs work by controlling the activation of this protein. However, drugs that activate multiple signaling pathways rather than a specific target pathway can lead to adverse side effects. Therefore, drug development focuses on activating specific molecular signal pathways within cells. Researchers from the University of Tokyo have discovered a new way of activating GPCR by triggering shape changes in the intracellular region of the receptor. This new process can help researchers design drugs with fewer or no side effects.
The Role of GPCRs in the Body
GPCRs are like snakes with seven segments traversing in and out of the cell membrane, acting as an inbox for messages. When a message molecule binds to the extracellular side of the receptor, it triggers a shape change activating G proteins and the ß-arrestin protein attached to the intracellular side of the receptor. The information then passes downstream and affects various bodily processes. GPCRs play a role in sensations such as light, smell, and taste.
The Need for Specificity in Drug Development
Drug development focuses on activating specific molecular signal pathways within cells to avoid adverse side effects. Activating the GPCR from inside the cell rather than outside the cell could be one way to achieve specificity. However, until now, there was no evidence of direct activation of only the intracellular side of GPCRs without the initiations from the extracellular side.
New Drug Design Strategies
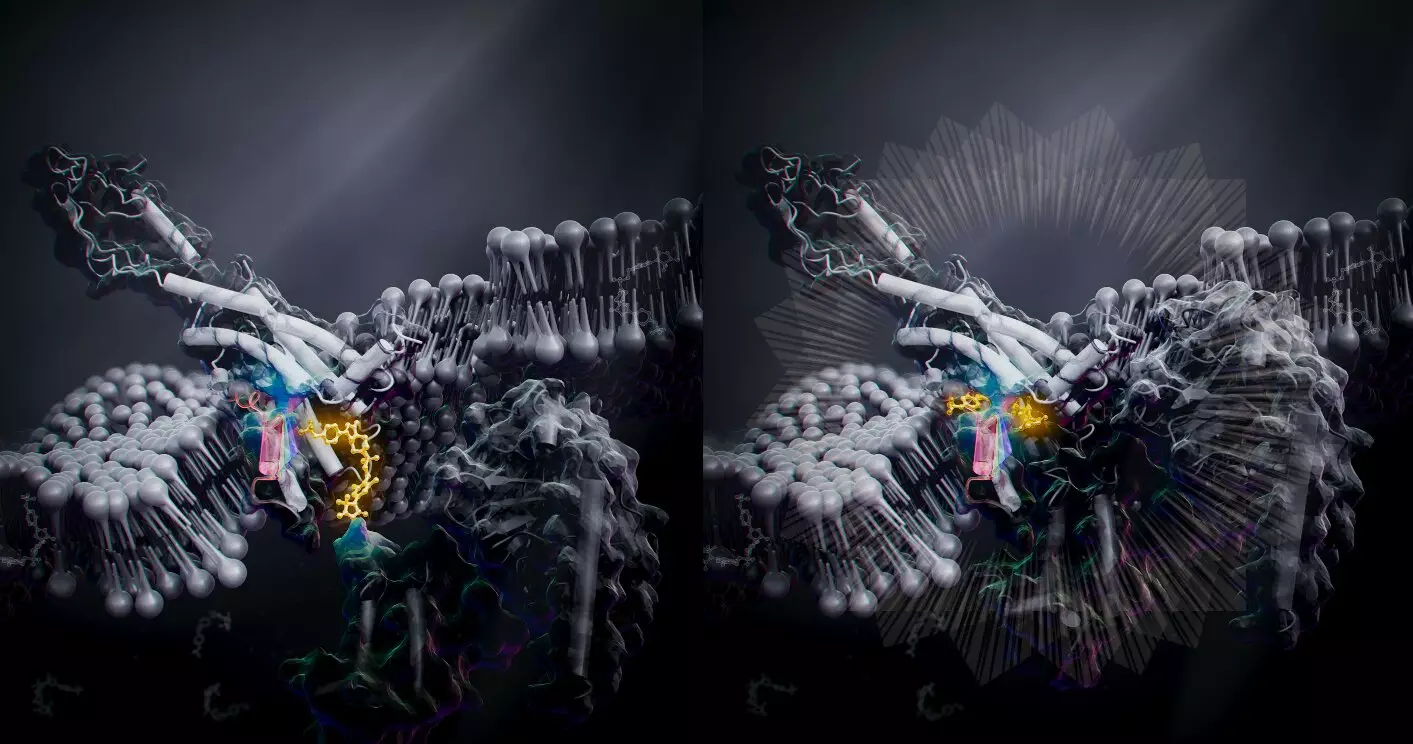
Researchers from the University of Tokyo conducted a study on bone metabolism-related GPCR called human parathyroid hormone type 1 receptor (PTH1R), which is a promising treatment for osteoporosis. The team used cryo-electron microscopy to reveal the 3D structure of the PTH1R and G protein bound to a message molecule. The team synthesized a non-peptide message molecule called PCO371 which binds to the intracellular region of the receptor and interacts directly with G protein subunits. In other words, PCO371 activates the receptor after entering the cell. The PCO371-bound PTH1R structure can directly and stably modulate the intracellular side of PTH1R. Because PCO371 activates only G protein and not ß-arrestin, it does not cause side effects. This specificity of its binding and receptor activation mode makes it a suitable candidate for potential small-molecule-based drugs for class B1 GPCRs, like PTH1R, which currently lack oral administrative drug ligands. Such drugs would have reduced adverse effects and burdens on patients as they act on specific molecular pathways.
The findings from this study will help researchers develop new drugs for disorders such as obesity, pain, osteoporosis, and neurological disorders. By triggering shape changes in the intracellular region of the receptor, drugs can achieve specificity, leading to fewer or no side effects. The discovery of PCO371 and its potential as a small-molecule-based drug for class B1 GPCRs is a promising step towards more effective drug development.

Leave a Reply