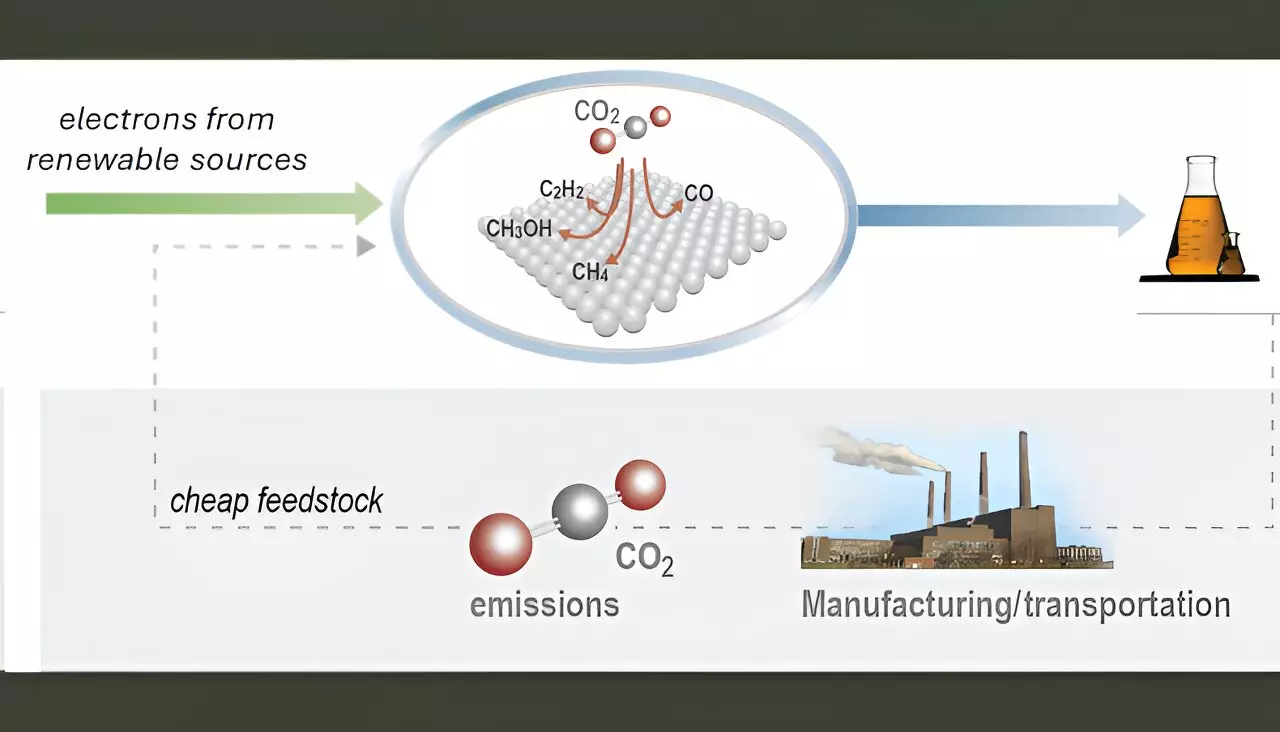
The multifaceted approach to mitigating climate change has crystallized around the ambitious goal of converting carbon dioxide (CO2), a notorious greenhouse gas, into valuable chemicals and fuels. This pivotal transformation can be powered through renewable energy sources like solar and wind, positioning CO2 not merely as a waste product but as a feedstock for the synthesis of high-demand chemicals essential in the chemical industry and transportation sectors. Products such as ethylene, ethanol, and acetic acid stand out as critical components derived from CO2 conversion, aiming to foster a sustainable industrial future.
Despite significant advancements in the design of electrolyzers—devices that harness electricity to facilitate chemical reactions—the commercialization of these technologies continues to face notable hurdles. A primary concern lies in the stability and selectivity of the catalysts employed in these systems. The quest for efficient and scalable catalysts is paramount, as these factors directly influence the overall performance and practicality of CO2 conversion technologies in real-world applications.
In response to the urgent need for better catalytic solutions, researchers at Lawrence Livermore National Laboratory (LLNL) have introduced an innovative catalyst coating platform utilizing physical vapor deposition (PVD). This sophisticated technique allows for exceptional control over numerous catalyst attributes, including thickness, composition, morphology, and porosity. Such granularity in design is vital when aiming to enhance the performance of copper-based catalysts, which have demonstrated potential in converting CO2 into multi-carbon products like ethylene and ethanol.
A significant impediment in catalyst development has been the challenge of disentangling the performance of the catalyst from the effects associated with its integration into electrolyzers. Variability in fabrication methods, electrolyzer configurations, and catalyst compositions can obscure performance metrics. To address this, the LLNL team has cultivated a catalyst platform that permits the tuning of catalyst composition while maintaining uniform morphology and integration profiles. This breakthrough offers a pathway to more accurately assess the intrinsic performance of various catalyst formulations.
The breakthrough research led by LLNL is not a solitary endeavor but rather a concerted effort involving collaboration with multiple academic institutions, including the University of Delaware, Washington University, and the University of Pennsylvania, as well as the industrial partner Twelve Benefits Corporation. This collaborative framework enriches the research, combining academic rigor with practical applications, each contributing unique insights and expertise toward the collective goal of advancing CO2 conversion technologies.
The LLNL team has notably employed a systematic approach to develop copper-based dilute alloy catalysts that are otherwise challenging to synthesize and integrate. The theoretical backing provided by advanced simulation techniques has enabled the research team to develop catalysts that effectively facilitate the coupling of carbon monoxide—an intermediate product in the electrolysis process—toward desired multicarbon outputs. These simulations not only guide the selection of materials but also enhance the understanding of the fundamental mechanisms at work during CO2 electrolysis.
The benefits of the PVD method extend beyond the controlled characteristics of the catalysts. PVD processes also result in less waste production compared to traditional electrodeposition techniques, marked by a reduction in labor intensity and, ultimately, a decrease in long-term operational costs. Although the initial capital outlay may be higher, the potential for using PVD to yield economically viable solutions for CO2 conversion in the chemical and transportation industries is unprecedented.
The evolution of CO2 electrochemical conversion technologies represents a promising frontier in the quest for sustainability. By effectively leveraging innovative catalyst designs and renewable energy sources, we might transform the chemical landscape into one that thrives on sustainability rather than exploitation. As interdisciplinary collaborations continue to burgeon, the realization of efficient CO2 conversion systems appears ever more attainable, paving the way for a sustainable future that reconciles industrial progress with environmental stewardship.

Leave a Reply