In the realm of organic chemistry, the synthesis of trisubstituted Z-alkenes has long posed significant hurdles due to their thermodynamic instability. Researchers from the National University of Singapore (NUS) have accomplished a remarkable feat by unveiling an iron-catalyzed technique that adeptly tackles this challenge. Their work, highlighted in the journal Nature Synthesis, underlines the pressing need for sustainable and efficient methods to produce these compounds, which are crucial for biologically active molecules and various stereospecific reactions.
Trisubstituted Z-alkenes are integral components of numerous biologically significant compounds. Their specific configuration is paramount for maintaining the integrity and functionality of various molecular structures commonly used in pharmaceuticals. However, the commonly encountered E-isomers are favored due to their greater stability, making the conventional approach to synthesizing Z-isomers both challenging and inefficient. The traditional methods often lack selectivity, which further complicates the synthesis of these important materials.
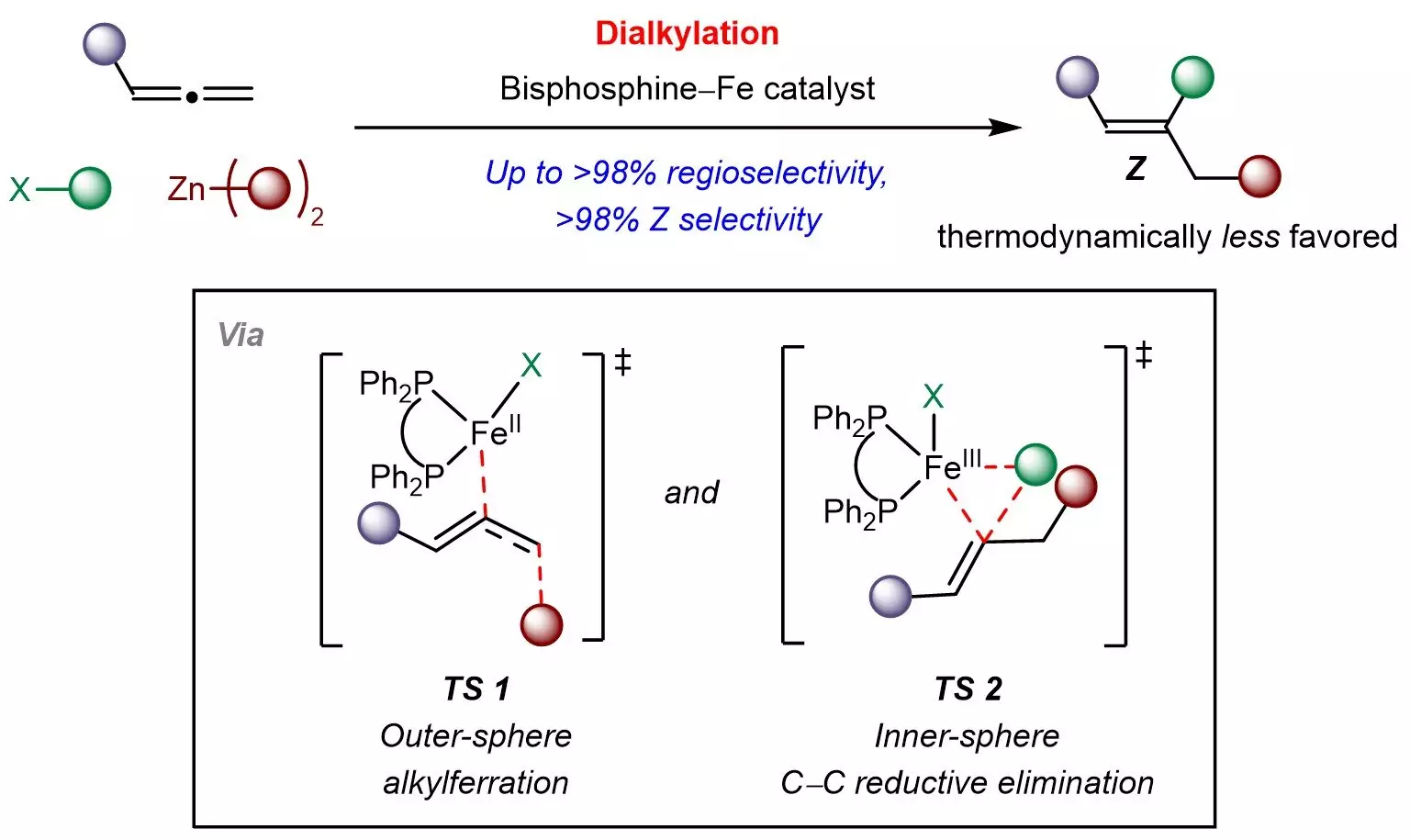
The research team, guided by Associate Professor Koh Ming Joo, has developed a novel multicomponent strategy that significantly enhances the formation of Z-alkenes. Utilizing an affordable bisphosphine-iron catalyst, they successfully coupled allenes with simple chemical building blocks like sp3-hybridized organohalides and organozinc reagents. This innovative approach not only facilitates the incorporation of various aliphatic groups but also allows for precise control over the site and selectivity of the Z-isomers.
This breakthrough is particularly relevant in the context of green chemistry, as the use of iron—a non-toxic and abundantly available transition metal—imparts both economic and environmental benefits to the synthetic process. Such advancements pave the way for a more sustainable approach to organic synthesis, with broader implications for the production of complex molecules.
One of the practical applications of this research is in the synthesis of a glucosylceramide synthase inhibitor, which necessitates a trisubstituted Z-alkene for its bioactivity. The method’s ability to streamline the production of such critical compounds open doors to further drug discovery efforts. Prof. Koh emphasizes the broader impact of their findings, stating that this method allows for meaningful exploration of these hard-to-acquire compounds in routine experimental settings.
Moreover, the research delves into a distinctive mechanism involving outer-sphere radical-mediated alkylferration followed by inner-sphere carbon-carbon bond formation. These insights not only elucidate the synthetic pathway but also inform future designs of kinetically controlled reactions involving allenes and other π-systems.
The findings from NUS signify a substantial leap in the pursuit of sustainable chemistry, addressing a critical gap in the literature pertaining to Z-alkene synthesis. As the team of chemists continues to explore additional multicomponent transformations, the potential for innovative applications across various industrial sectors is immense. By transforming abundant raw materials into value-added products, this research sets the stage for a new era in organic synthesis that aligns with the principles of sustainability and efficiency.

Leave a Reply