In the pursuit of sustainable energy solutions, hydrogen is often hailed as a game-changer. Its potential for powering vehicles, homes, and industries is significant, yet one major hurdle remains: storage. Researchers from the Leibniz Institute for Catalysis (LIKAT) in Rostock, in collaboration with H2APEX, have developed a novel method to safely and stably store hydrogen, illuminating a path forward for its use in energy systems. Their groundbreaking study, published in Nature Communications, unveils a method that combines simplicity with effectiveness, utilizing commonly known substances to create an efficient hydrogen storage medium.
At the heart of this innovative research lies a special catalyst system that enables the chemical binding of hydrogen (H2) to potassium bicarbonate, a compound that is not only safe but also familiar to many as a cooking ingredient—baking soda. This process leads to the formation of formate, a benign salt derived from formic acid. Researchers Dr. Rui Sang and Ph.D. student Carolin Stein detail the genius of the approach, which allows for reversible reactions; hydrogen can be stored by binding it chemically in the formate, and can subsequently be released whenever needed, using the same catalyst and system.
The use of a ruthenium-based catalyst, which is widely available and relatively easy to produce, facilitates this reaction. The stability of the system at approximately 60°C further demonstrates its practicality and efficiency. Unlike conventional storage methods, this chemical reaction occurs within a liquid solution that contains all requisite elements, including hydrogen, bicarbonate, and the catalyst. Such a design minimizes complications and enhances usability.
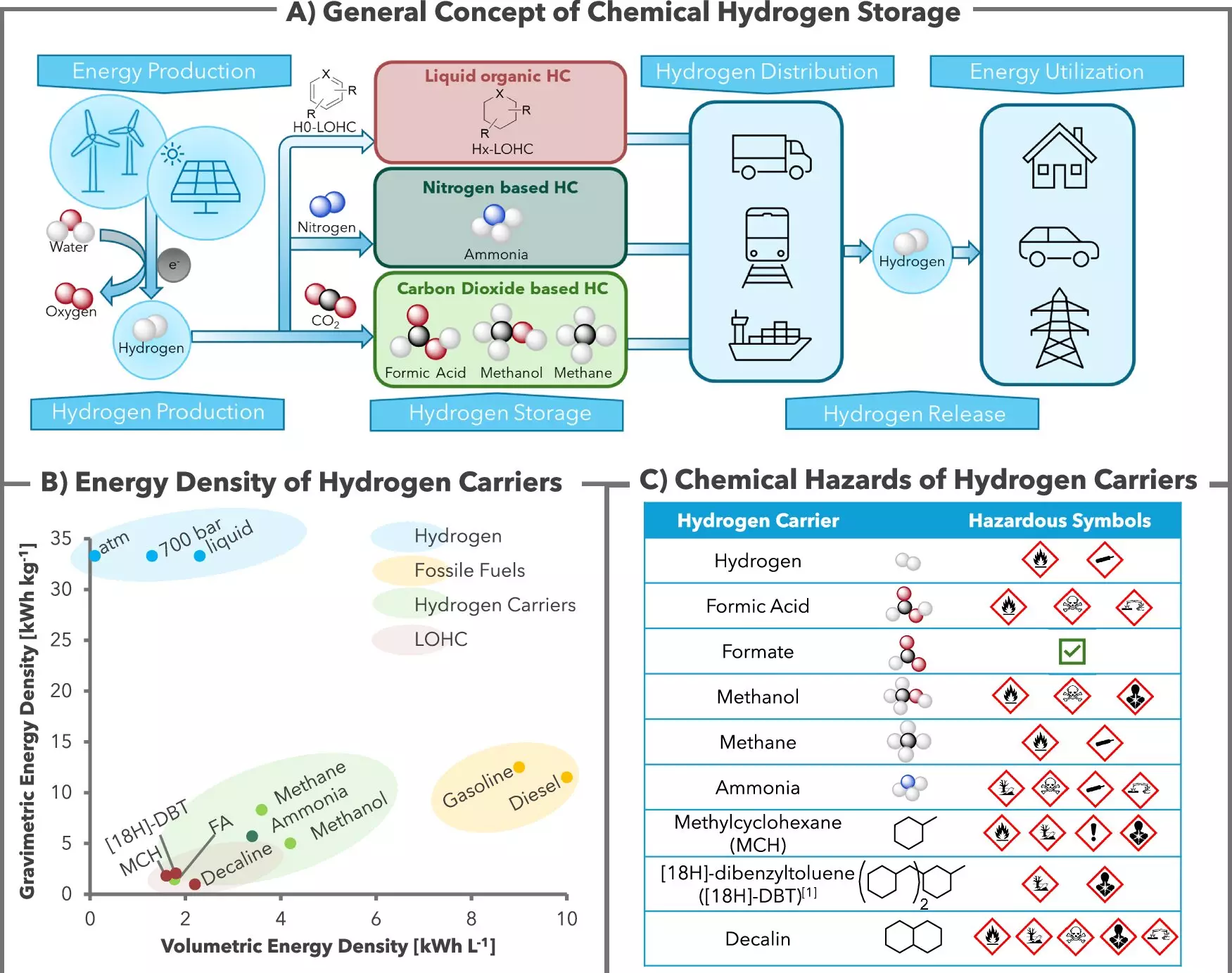
The authors of the study point out that formic acid salts present numerous advantages over other potential hydrogen storage media such as methanol, ammonia, and methane. A significant factor is their superior safety profiles and lower toxicity, making them far more suitable for widespread deployment. Additionally, the formate can be transported in standard plastic containers and tankers—akin to how perishable goods are moved today—thereby lowering logistical barriers often encountered in the energy sector.
Moreover, the formate-bicarbonate system resembles a battery in that it offers a reversible charge and discharge mechanism. Solar or wind energy can convert into hydrogen through electrolysis during periods of surplus electricity, which can then be converted into formate for storage. This ability to effectively harness renewable energy could be transformative, particularly for rural or underserved areas that are often the first to adopt new technologies.
Importantly, this hydrogen storage process is CO2-neutral, a factor that cannot be overstated in an era where climate change looms large. While traditional methods often release CO2 into the atmosphere during hydrogen recovery, this innovative method effectively confines the CO2, allowing for the production of pure hydrogen that can be directly utilized in fuel cells without additional processing. This means that the end result is not just a technical advancement but also a step forward in environmental sustainability.
As the researchers have successfully demonstrated 40 cycles of hydrogen storage and release over six months, generating over 50 liters of high-purity hydrogen, the practical implications are promising. The ongoing collaboration with H2APEX aims to translate these findings into larger, commercially viable applications by 2025, further bridging the gap between laboratory research and real-world solutions.
Ultimately, the innovation presented by the LIKAT and H2APEX researchers represents a pivotal moment in the ongoing quest for clean, renewable energy solutions. The implications of this work could reverberate throughout various sectors, leading to more efficient hydrogen energy systems that are not only safe but accessible. As the industry moves toward commercialization, this new method could pave the way for hydrogen to become a cornerstone of our future energy landscape—bringing with it the promise of hope, progress, and sustainability in the transition to a greener world.

Leave a Reply