In the realm of scientific innovation, the quest for self-assembly has intrigued researchers for decades. Supramolecular chemistry stands at the forefront of this exploration, focusing on how large, complex structures can be constructed from smaller, discrete components. This field holds the key to creating “smart materials”—substances capable of responding dynamically to environmental cues—a concept that could revolutionize industries from biomedicine to materials science.
Understanding self-assembly is crucial, especially considering its prevalence in biological systems. From proteins and cell membranes to viruses, nature exhibits an intricate design where simple building blocks unite to form sophisticated structures. However, despite substantial advances, many aspects of supramolecular interactions remain enigmatic, leaving much room for discovery.
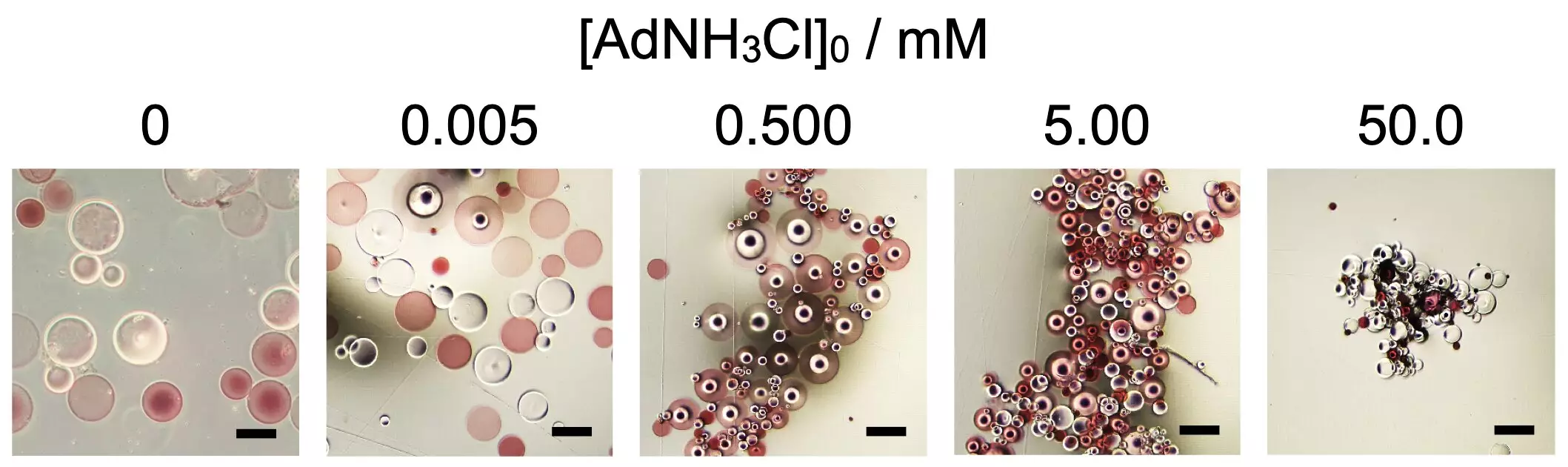
A recent study from Osaka University sheds light on how certain additives can facilitate the self-assembly process of spherical microparticles derived from poly(sodium acrylate), a widely-used super absorbent polymer. By exploring different functionalizations, researchers strategically introduced β-cyclodextrin (βCD) and adamantane (Ad) residues to the polymer chains. Surprisingly, the assembly only began when a specific concentration of 1-adamantanamine hydrochloride (AdNH3Cl) was introduced, emphasizing the precise control additives have over assembly processes.
This discovery parallels the behavior of biological proteins, which rely on interactions among amino acids to determine their folded shapes. Hydrogen bonds, electrostatic forces, and hydrophobic interactions play critical roles in shaping a protein’s final form, mirroring the ways that additives in this study influenced the polymer assemblies. The work of researchers, particularly the lead author Akihito Hashidzume, offers insights into the complex interactions that dictate not only molecular behavior but also broader biological phenomena.
The implications of this research extend far beyond mere curiosity. By demonstrating that variation in additive concentration can ensure the control of macroscopic assembly shapes—whether they appear more spherical or elongated—scientists are paving the way for advanced applications. This ability to control structure and shape through fine-tuning of molecular interactions suggests exciting possibilities in creating responsive materials that adapt to changes in their environment, from heat to mechanical stress.
Senior author Akira Harada notes that these findings may also illuminate the origins of various forms found in biological organisms. The parallels drawn between the structure-making capabilities of polymers and the complex arrangements seen in nature could lead to new understandings of morphological design, potentially impacting fields such as synthetic biology and tissue engineering.
The intersection of supramolecular chemistry and the natural process of self-assembly offers a promising frontier for scientific exploration. As researchers unravel the intricacies of molecular interactions, the development of smart materials that can react adaptively to their surroundings becomes increasingly feasible. This burgeoning area of study not only enhances our understanding of fundamental biological processes but also sets the stage for innovative applications that could redefine what materials can do, fundamentally changing technology as we know it. Thus, the ongoing journey into the world of supramolecular chemistry remains as exciting and relevant as ever, full of potential for shaping the future.

Leave a Reply