A pivotal study spearheaded by Professor Jaeheung Cho and his team at UNIST’s Department of Chemistry has recently emerged in the Journal of the American Chemical Society. Their groundbreaking research investigates the intricate reactions between cobalt(III)-based metal complexes and nitrile compounds, thereby laying the foundation for innovative approaches in drug discovery. Cobalt(III) complexes offer a fertile ground due to their unique chemical properties, making them significant in the realm of medicinal chemistry.
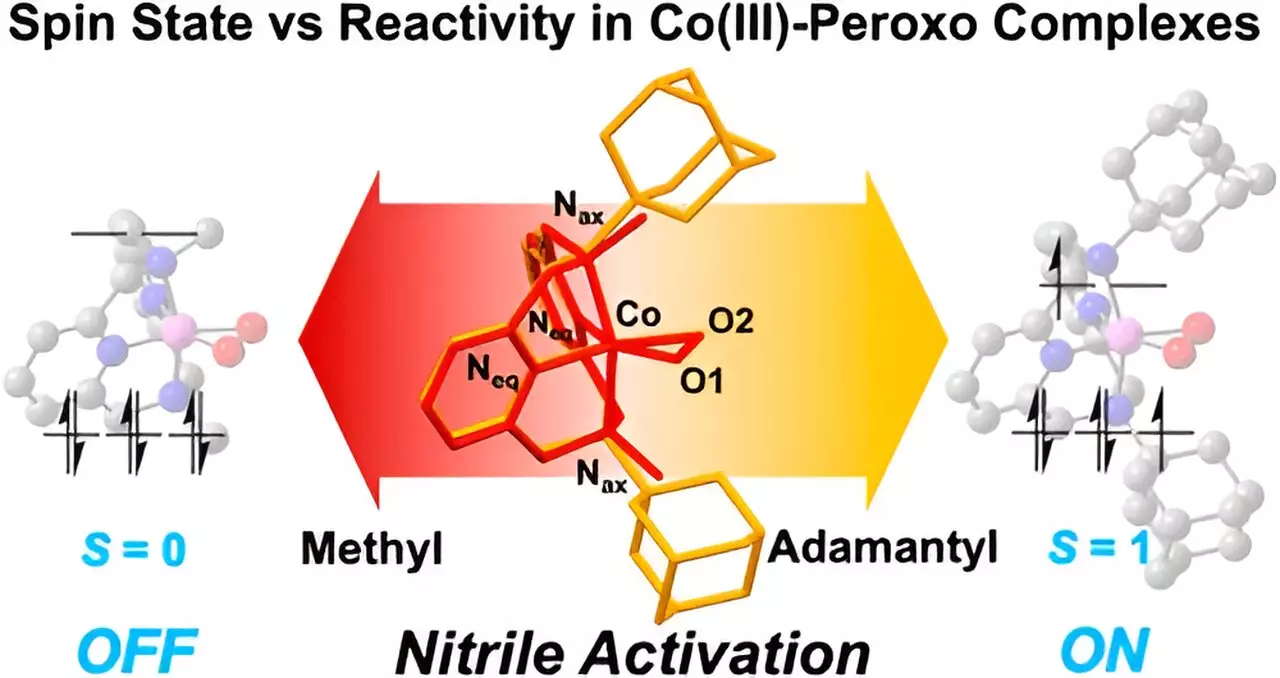
The research focused on understanding the mechanisms by which nitriles interact with these cobalt complexes. By leveraging a sophisticated framework known as the Macrocyclic Pyridinophane System, the researchers could manipulate the physical configuration of the cobalt compounds. This manipulation was crucial in determining how easily nitriles could be activated. Importantly, the study highlighted that reaction rates and outcomes are dramatically influenced by the spin states of the metal—an aspect that is often overlooked in similar investigations.
The findings are particularly striking, as the team observed that even minute alterations in the metal structure can lead to significantly different reactivity profiles. For instance, cobalt compounds with larger adamantyl groups demonstrated a marked increase in nitrile activation compared to those with smaller methyl groups, which remained inert. This variation emphasizes the importance of the metal spin states, which dictate how these complexes interact with nitrile compounds.
The implications of these findings are far-reaching, especially in pharmaceuticals and agrochemicals, where nitriles are extensively utilized. However, the inherent reactivity issues associated with nitriles have often hindered their function in drug development. The research team’s breakthrough in confirming that cobalt(III)-peroxo species can effectively react with nitriles at room temperature is a significant advancement. The synthesized compounds appear to show promise as potential anticancer agents, marking a crucial step toward addressing the longstanding challenges associated with nitriles in the pharmaceutical industry.
First author Seonghan Kim articulated the relationship between ligand modifications and nitrile reactivity, stating, “We successfully synthesized cobalt(III)-peroxo species with varying spin states by modifying the three-dimensional configuration of the ligands.” This insight reveals a crucial connection between the structural aspects of metal complexes and their reactivity, which could have profound implications for designing more effective drugs. The ability to tailor reactions through structural control opens up a new avenue for researchers aiming to optimize the functionality of metal complexes in medicinal applications.
The research conducted by Cho and his team not only elucidates the complex interactions between cobalt(III) compounds and nitriles but also sets the stage for future developments in drug creation. As the scientific community continues to explore the depths of metal-centered chemistry, this study will likely inspire further investigations into efficient and effective drug development strategies, potentially revolutionizing how medicinal compounds are designed and synthesized. The integration of structural chemistry with metal reactivity presents an exciting frontier that could yield transformative results in therapeutic applications.


Leave a Reply