High entropy oxides are gaining significant attention due to their unique structural and functional properties, making them potential candidates for various applications, particularly in electronic devices. A recent study sheds light on how different synthesis methods can drastically influence these properties. Published in the Journal of the American Chemical Society, this research expands our understanding of material science and highlights the delicate interplay between synthesis techniques and resulting material characteristics.
At the heart of this investigation is a high entropy oxide characterized by a spinel crystal structure, composed of a blend of five transition metal oxides. Alannah Hallas, a materials scientist from the University of British Columbia’s Blusson Quantum Matter Institute, emphasized that the excitement surrounding this class of materials stems from their enhanced electrochemical properties. High entropy oxides possess an extraordinary level of chemical flexibility, offering researchers a multitude of avenues to tailor these materials for specific applications. The ability to manipulate various synthesis parameters provides a canvas for innovative material design.
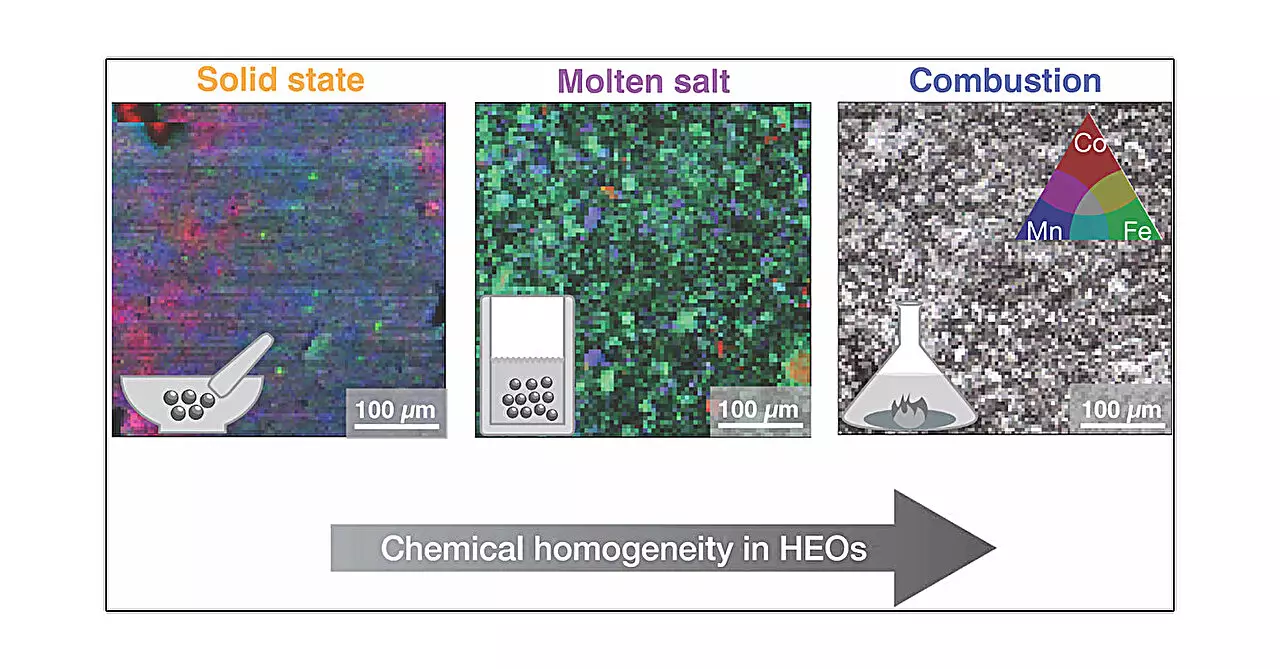
The research team employed five distinct synthesis methods: solid state, high pressure, hydrothermal, molten salt, and combustion. Each method presents unique thermal and chemical conditions that affect material formation. For instance, the solid-state method resembles baking, where metal oxides are mixed and heated. In contrast, the high-pressure method applies external pressure, influencing the crystallization process. The hydrothermal approach simulates conditions found in nature, enabling crystal growth in a pressurized water environment. Meanwhile, molten salt synthesis allows for a controlled precipitation of crystals from a thick liquid as it cools down. Lastly, combustion synthesis utilizes a rapid exothermic reaction to generate the desired materials quickly.
The lead author of the study, Mario Ulises Gonzáles-Rivas, brings his expertise in these synthesis techniques, having perfected the art of sample preparation during his doctoral research. His insights reveal that while the average structure of the synthesized materials remains unchanged across different methods, significant differences in their local structures and microstructural properties emerge.
One of the crucial findings from this study is the strong impact that the chosen synthesis method has on the final material characteristics. Although the overarching structure appears stable, the local structural variations can dictate functionality. The combustion method, in particular, yielded the most homogeneous samples, indicating its potential advantages in achieving uniform material properties. This suggests that synthesis technique is not merely a procedural step but a vital component influencing the overall performance and usability of high entropy oxides in practical applications.
The ramifications of this study extend beyond academic knowledge to real-world applications. These high entropy materials show promise in addressing critical energy challenges, from battery technologies to catalysis. Gonzáles-Rivas articulated that the nuanced structural variations observed could have vital implications for the technological integration of these materials. By providing insights into optimal synthesis pathways, researchers can more effectively harness the capabilities of high entropy oxides in energy systems.
This research is a testament to collaborative scientific inquiry, involving contributions from Hallas’s team at UBC, Robert Green from the University of Saskatchewan, and Hidenori Takagi at the Max Planck Institute for Solid State Research. Such interdisciplinary partnerships are crucial in advancing our understanding of complex materials and driving innovations that can lead to novel applications.
The study of high entropy oxides and their synthesis methods reveals a fascinating interplay between material science and practical applications. The ability to optimize synthesis techniques not only enhances our understanding of material properties but also accelerates the development of next-generation materials for electronic and energy applications. As research continues to evolve in this field, the potential for high entropy oxides to contribute to future technological advancements appears promising, paving the way for enhanced performance in various sectors.

Leave a Reply