In a groundbreaking study spearheaded by a team at Rice University, researchers have begun to unravel the complex interplay between cholesterol and cell membranes. Led by Jason Hafner, a professor specializing in physics and chemistry, the findings present a significant advance in our comprehension of how cholesterol shapes these vital structures. The study, recently published in the Journal of Physical Chemistry, emphasizes cholesterol’s critical function in organizing cell membranes, which are intricate assemblies of proteins and lipids that facilitate various cellular processes.
Cholesterol has long been acknowledged for its role within biomembranes, but studying its behavior and interactions has posed considerable challenges. Hafner highlighted the potential impact of their research, particularly concerning diseases like cancer where membrane integrity and organization are paramount. As the structure of cell membranes affects many physiological responses including receptor functions, understanding cholesterol’s contributions could pave the way for new therapeutic approaches.
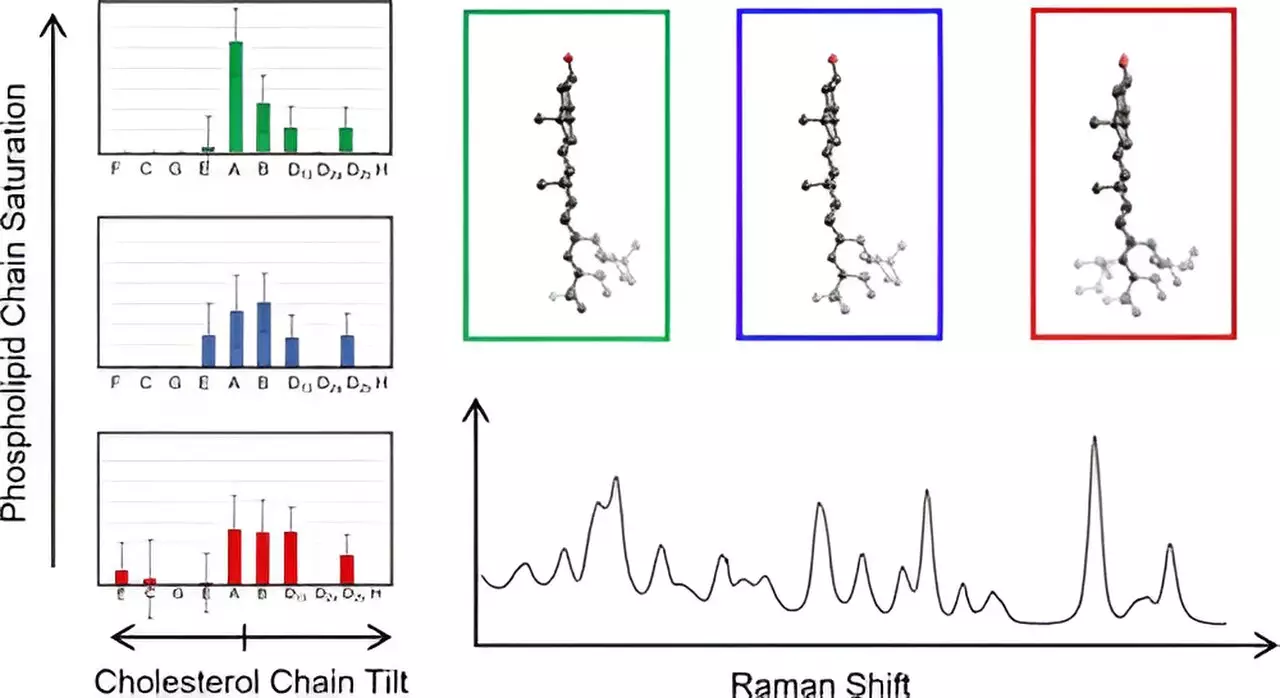
At the heart of this research lies an advanced technique known as Raman spectroscopy. This method employs laser light to probe the molecular structures within membranes, generating intricate vibrational spectra that reveal essential information about molecular interactions. Hafner’s team adopted this approach to investigate cholesterol molecules situated in natural membrane environments. By comparing the collected spectra with theoretical predictions derived from density functional theory—a sophisticated quantum mechanical tool—they gleaned new insights into cholesterol’s structural variations.
The research involved an extensive analysis of 60 different cholesterol arrangements, meticulously focusing on its distinctive fused ring structure accompanied by an eight-carbon chain. Through systematic exploration, the researchers identified that variations in the orientation of this chain—specifically how it deviates from the plane of the sterol rings—could categorize the cholesterol molecules into groups based on their vibrational characteristics. This categorization not only provided clarity about the previously overlooked structural distinctions but also simplified data interpretation.
One of the unexpected findings from this study was the discovery that cholesterol molecules sharing similar group characteristics exhibited identical spectra at low frequencies. This was a pivotal moment that enabled the research team to streamline their analytical processes and construct an accurate mapping of cholesterol chain structures within membranes. Such insights could revolutionize the understanding of membrane biophysics and enhance knowledge regarding various diseases linked to membrane organization.
The contributions of this research extend beyond mere academic curiosity; integrative studies like this one can significantly influence future biomedical research and therapeutic strategies against diseases where membrane organizations are compromised. Alongside Hafner, the team included graduate and undergraduate students, showcasing a collaborative effort that enriches educational experience and research innovation.
In essence, as researchers continue to navigate the intricate world of cell membranes, the revelations about cholesterol’s role not only enhance scientific understanding but also herald a future where targeted medical interventions may improve health outcomes in diseases associated with membrane dysfunction.


Leave a Reply