The Bipartisan Infrastructure Law has recently identified the need to capture carbon dioxide (CO2) and transport it for permanent storage or conversion into valued end uses to move toward reaching net-zero greenhouse gas emissions by 2050. A team of researchers from Northwestern University has collaborated with other international researchers to create acetic acid out of carbon monoxide derived from captured carbon. The innovation could potentially spur new interest in carbon capture and storage.
Electrochemistry for Carbon Capture
Northwestern University professor Ted Sargent and his team have a history of using electrolyzers to convert captured carbon into key industrial chemicals, including ethylene and propanol. In this research, they have created a novel catalyst that uses electrochemistry to convert captured carbon into products with established markets. This provides new pathways to improve the economic feasibility of carbon capture. Acetic acid production is the newest successful venture of this team.
Acetic Acid Production
Acetic acid is a key component in household vinegar, but this accounts for only a small proportion of its use. The majority of the acetic acid market is for feedstock in the manufacture of paints, coatings, adhesives, and other products. Currently, production at this scale is primarily derived from methanol, which comes from fossil fuels. However, lifecycle assessment databases have shown that for every kilogram of acetic acid produced from methanol, the process releases 1.6 kg of CO2.
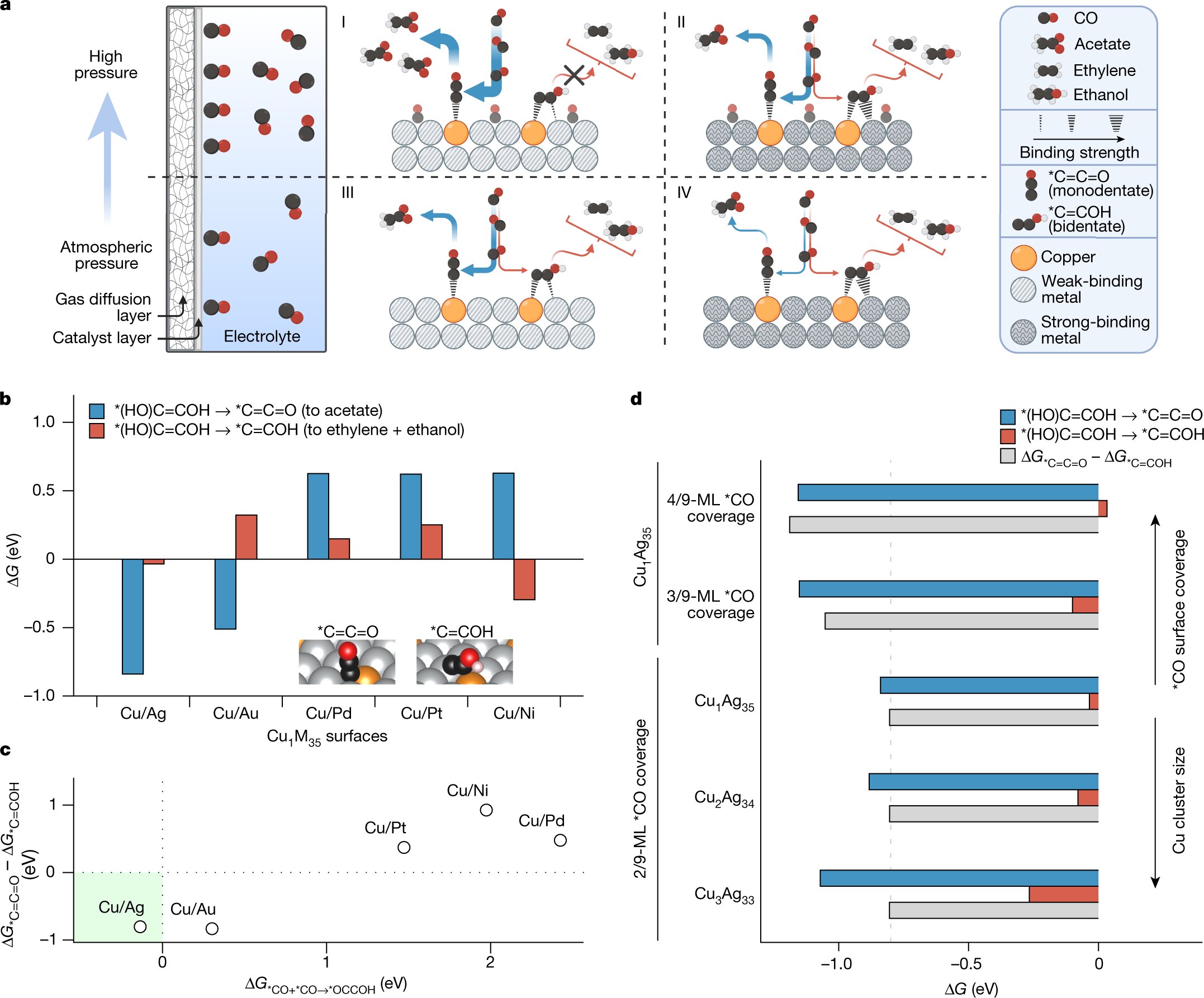
The team’s alternative method for acetic acid production takes place via a two-step process. First, captured gaseous CO2 is passed through an electrolyzer, where it reacts with water and electrons to form carbon monoxide (CO). Gaseous CO is then passed through a second electrolyzer, where another catalyst transforms it into various molecules containing two or more carbon atoms. The team has set up conditions that favor the production of acetic acid above all other two-carbon products.
Efficiency and Stability of the Catalyst
The team’s analysis has shown that using a much lower proportion of copper (approximately 1%) compared with previous catalysts would favor the production of just acetic acid. It also showed that elevating the pressure to 10 atmospheres would enable the team to achieve record-breaking efficiency. In the paper, the team reports a faradic efficiency of 91%, meaning that 91 out of every 100 electrons pumped into the electrolyzers end up in the desired product—in this case, acetic acid. The new catalyst also appears to be relatively stable, as it remained at a high level of 85% even after 820 hours of operation.
This new method of using electrochemistry to convert captured carbon into acetic acid provides a more sustainable source for the industrial chemicals that we still need. The team’s success in creating a novel catalyst and optimizing the production process could inspire other teams to think outside the box and develop additional pathways to useful products, including ethanol, propylene, and other multi-carbon products. As we move towards a decarbonized chemical industry, having various sustainable pathways is crucial.

Leave a Reply