The increasing use of electric and hybrid vehicles has underscored the need for safe and high-performing battery technologies. Engineers have been striving to enhance the safety, energy capacity, scalability, and longevity of batteries. One of the promising options for meeting the demands of the electronics industry is rechargeable multivalent metal batteries. These batteries employ multivalent ions and anode materials like magnesium (Mg) and calcium (Ca), which have low-reduction potentials. By utilizing the right combination of anodes, cathodes, and electrolytes, these batteries can achieve high energy densities.
Recently, a team of researchers from Zhejiang University, the ZJU-Hangzhou Global Scientific and Technological Innovation Center, and Dalian University of Technology introduced a universal approach to develop highly performing and scalable electrolytes for multivalent metal batteries. Their strategy, outlined in a paper published in Nature Energy, aims to create reversible and affordable electrolyte systems that can propel next-generation battery technologies.
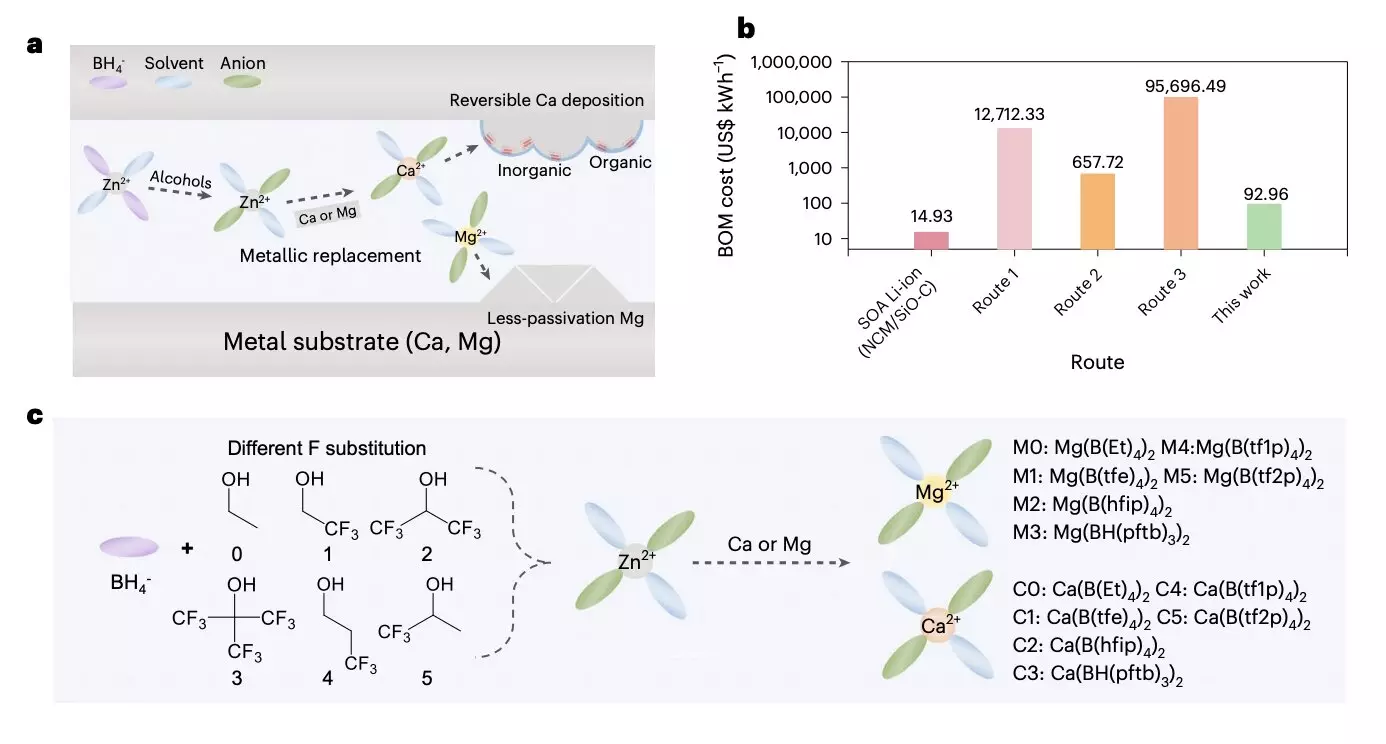
The researchers acknowledged the demand for high-performance and cost-efficient electrolytes for multivalent metal batteries. However, the expensive precursor and complex synthesis processes have hindered the exploration of cathode electrode/electrolyte interfaces and solvation structures. To address these challenges, the team developed a universal cation replacement method to prepare low-cost and highly reversible magnesium and calcium electrolytes derived from a zinc organoborate solvation structure.
The researchers followed a series of steps to implement their method. They initiated a chemical reaction between an easily attainable Zn(BH4)2 precursor and various fluoroalcohols to generate anions with different branched chains. These anion solvates then reacted with low-cost metal foils with higher metal activity to produce target solvation structures. Additionally, to ensure stable battery cycling and suppress solvent decomposition, the researchers proposed the formation of a passivation layer based on two types of calcium solvates.
The team explained that by fine-tuning the precursor chain length and F-substitution degree, they could effectively regulate anion participation in the primary solvation shell. The completely dissociated magnesium organoborate electrolyte exhibited high current endurance and enhanced electrochemical kinetics. On the other hand, the calcium organoborate electrolyte with strong coordination and B–H inclusion offered a stable solid-electrolyte interphase with high coulombic efficiency.
The researchers utilized their method to create a high-loading battery prototype based on Mg/S, which boasted a specific energy of 53.4 Wh/kg. This prototype consisted of a 30 μm Mg anode, a low electrolyte/sulfur ratio (E/S = 5.58 μl/mg), and a modified separator/interlayer. Initial tests of the battery prototype yielded promising results, demonstrating the potential of this approach to generate favorable and low-cost electrolytes for multivalent metal batteries.
Looking ahead, the method introduced by this research team holds promise for developing various reversible electrolyte systems that rely on more affordable materials and simpler processing strategies. These scalable and safe electrolytes could contribute to the creation of multivalent metal batteries with higher energy densities. Furthermore, the widespread adoption of such batteries could pave the way for advancements in electric and hybrid vehicles, as well as other energy storage applications.
In an era when the demand for electric and hybrid vehicles is rapidly growing, the development of safe and high-performing battery technologies is paramount. The research conducted by the team from Zhejiang University, the ZJU-Hangzhou Global Scientific and Technological Innovation Center, and Dalian University of Technology is a significant step forward in addressing these needs. By introducing a new method for scalable and affordable electrolytes for multivalent metal batteries, they have opened up possibilities for next-generation battery technologies that can revolutionize the electronics industry. With further research and development, these electrolytes could play a crucial role in powering the vehicles and devices of the future.


Leave a Reply