As the global shift towards greener power sources gains momentum, the focus on transitioning from gas to electricity extends beyond just vehicles. Powering the vast manufacturing network responsible for producing essential products, from batteries to fertilizers, also needs to embrace sustainability. In a recent study published in Nature Catalysis, UChicago chemists have made significant progress in the field of electrochemistry. By harnessing the power of electricity, they have found a way to enhance chemical reactions used in the synthesis of potential pharmaceutical drugs. This breakthrough opens avenues for designing, controlling, and ultimately making chemical reactions more sustainable.
Unraveling the Complexities of Electrochemistry
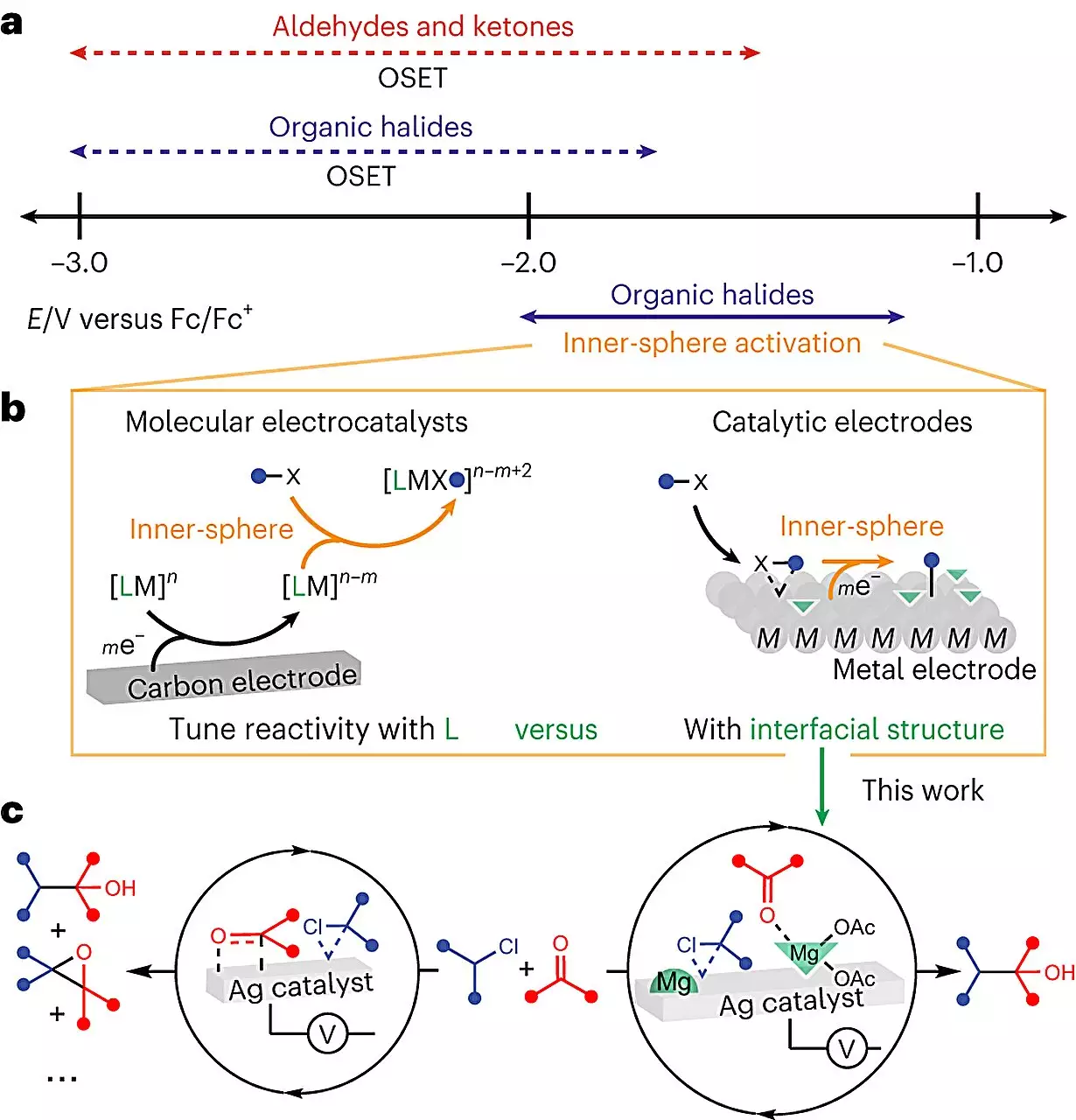
The world of electrochemistry is exceptionally intricate, posing numerous challenges to scientists attempting to understand molecular interactions and optimize reaction efficiency. One of these challenges arises from the need to introduce a conductive solid, known as an electrode, to provide the required electricity. Consequently, the molecules not only interact with one another but also with the electrode. UChicago’s Neubauer Family Assistant Professor, Anna Wuttig, aims to convert this complexity into an advantage. By leveraging electrochemistry’s unique design lever, she believes it is possible to achieve breakthroughs not attainable in any other system.
Wuttig and her team directed their efforts towards exploring the catalytic potential of the electrode’s surface in a commonly used reaction for manufacturing medicinal chemicals. The objective was to form a bond between two carbon atoms, which theoretical predictions suggested could achieve a 100% yield when powered by electricity. However, actual lab experiments fell short of this goal. The team hypothesized that the presence of the electrode was diverting some molecules away from their desired location during the reaction. Through extensive experimentation, they discovered that the inclusion of a Lewis acid, a chemical additive, redirected the molecules, resulting in a significantly higher yield. The addition of this “modulator” led to near-clean reactions, reducing unwanted byproducts and enhancing the overall efficiency.
One of the crucial advancements in this study was the application of special imaging techniques that allowed the team to visualize and understand the molecular processes occurring in real-time. By observing the reactions at the interfacial level, they were able to ascertain the profound impact of the modulator on the reaction’s structure. This breakthrough not only demystifies the “black box” nature of electrochemical reactions but also paves the way for predicting and controlling their effects. Furthermore, the electrode can be reused in subsequent reactions, adding another layer of sustainability to the synthesis process.
Towards Sustainable Synthesis
The implications of the UChicago study extend far beyond the realm of electrochemistry. By demonstrating the potential for enhanced reaction efficiency and control through electrode surface catalysis, this research offers a sustainable path forward in chemical synthesis. The ability to reduce waste, utilize renewable energy sources, and predict reaction outcomes brings us closer to a greener and more environmentally conscious global manufacturing network. The field of electrochemistry holds significant promise as a key ally in our quest for sustainable development.
With the transition from gas to electricity gaining momentum worldwide, the need to transform the manufacturing network becomes evident. UChicago’s breakthrough research in electrochemistry brings us closer to sustainable synthesis processes. By utilizing electricity to boost chemical reactions and exploring the catalytic potential of the electrode’s surface, significant strides towards efficiency and control have been made. The visualization and understanding of molecular interactions within these electrochemical reactions enable scientists to predict and optimize outcomes. As we navigate towards a greener future, harnessing the power of electrochemistry offers a promising solution in our pursuit of sustainability.

Leave a Reply