The production of hydrogen through water splitting using solar energy or other renewable resources is seen as a promising method for sustainable hydrogen production on a large scale. However, most photoelectrochemical water splitting systems currently available are either inefficient, unstable, or not suitable for large-scale implementation. Researchers at the Ulsan National Institute of Science and Technology (UNIST) have recently developed an innovative and scalable photoelectrochemical (PEC) system for the production of green hydrogen. Their system is based on a formamidinium lead triiodide (FAPbI3) perovskite-based photoanode encapsulated by a nickel foil and an NiFeOOH electrocatalyst. This article will delve into the details of their research and discuss the potential implications of this breakthrough.
Achieving a minimum of 10% solar-to-hydrogen (STH) efficiency is crucial for the development of a practical PEC system. Previous attempts at photoelectrochemical hydrogen production have utilized metal oxides as photoelectrode materials. These systems, however, have not yielded the necessary efficiencies for practical applications. Researchers have turned to photovoltaic (PV) grade materials, such as silicon, perovskites, chalcogenides, and III-V material classes, for potential high-efficiency photoelectrodes. Metal-halide perovskites (MHPs), in particular, show promise due to their unique characteristics of high efficiency and low cost. However, their stability in humid conditions and under UV light poses a significant challenge.
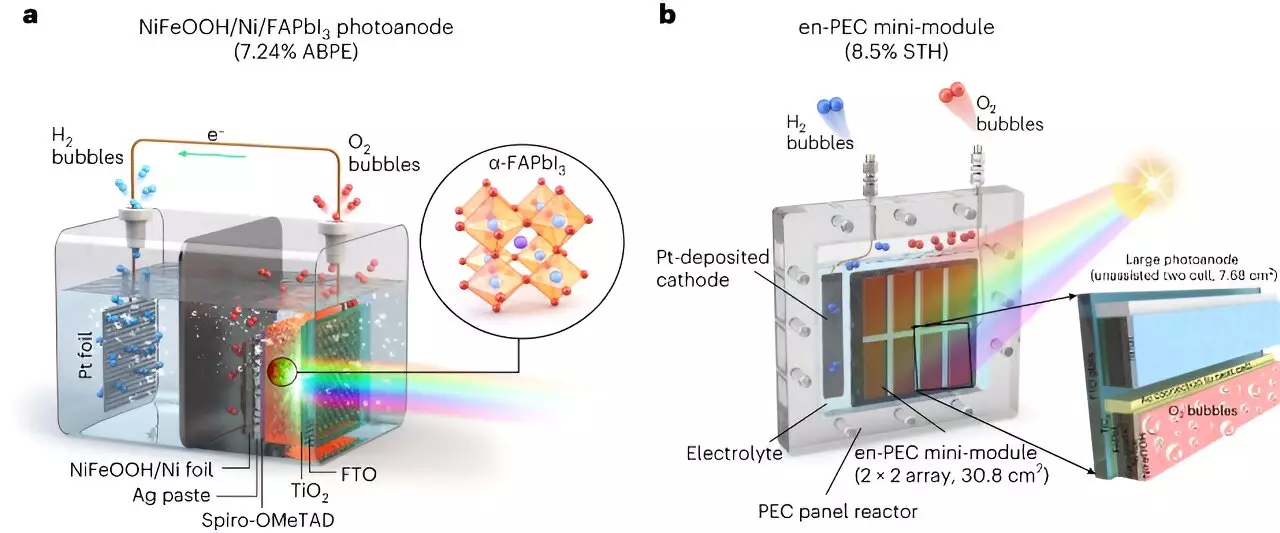
To overcome the stability challenge associated with MHPs, the researchers at UNIST aimed to stabilize these materials using metal-encapsulation or metal-protection techniques. They also utilized the UV-stable FAPbI3 perovskite to enhance stability. The selection of a stable and efficient material was crucial for the success of the proposed PEC system. The researchers encapsulated the FAPbI3 perovskite with a thick nickel foil (30mm) and deposited an NiFeOOH catalyst on top to protect the perovskite and promote the oxygen evolution reaction for water splitting.
The researchers initially developed a small-scale version of their proposed PEC system, which achieved a 9.89% STH efficiency and demonstrated long-term stability. They then scaled up the system using a module-based design. The basic mini-module consisted of a 7.68 cm2 device, and multiple mini-modules were combined horizontally and vertically to fabricate a large-scale device. Remarkably, the scaled-up system maintained its efficiency and stability, suggesting that the design is highly scalable.
The all-perovskite PEC system developed by the researchers at UNIST consists of an FAPbI3 photoanode encapsulated with a nickel metal foil as an encapsulation layer and an NiFeOOH catalyst layer. This photoanode is optimized with different metal foils, and the interactions between the catalyst and electrolyte are thoroughly studied. In the PEC device, the photoanode is connected in parallel to another MHP thin film as a photovoltaic (PV) unit cell to generate sufficient voltage for water splitting. The integration of both components simplifies the overall system, reducing complexity and lowering fabrication costs.
The mini-modules developed by the researchers are arranged in a 4 x 4 array, combining the photoelectrode and PV unit cell. This integrated system eliminates the need for additional PV components, making it more practical and cost-effective. Even when deployed on a larger scale, the system retains its performance and stability. The successful demonstration of a scalable PEC system is a significant step towards practical applications of photoelectrochemical technology for green hydrogen production in outdoor conditions.
The researchers at UNIST are committed to further improving the efficiency and stability of the PEC system by integrating more efficient and durable catalysts and selecting optimal photoelectrodes. Their research has laid the groundwork for the development of sustainable hydrogen production methods that can contribute to a greener and more sustainable future.
The development of a scalable and efficient PEC system for hydrogen production is a crucial step towards sustainable energy solutions. The use of MHPs and the encapsulation techniques employed by the researchers at UNIST offer promising possibilities for practical and cost-effective hydrogen production. As further advancements are made in the field of photoelectrochemical water splitting, the dream of widespread renewable hydrogen production may become a reality.

Leave a Reply