Researchers at Case Western Reserve University are making significant strides in their efforts to convert waste into fuels and other valuable products using energy-efficient and renewable processes. Their focus lies specifically in tackling the challenge of converting carbon dioxide (CO2), a prominent greenhouse gas, into useful chemicals through the use of electricity.
Converting CO2 into commodity chemicals and fuels is a complex process that necessitates high pressures, high temperatures, and specialized materials. Finding a solution has become increasingly important given the pressing need for technologies that can capture CO2 from waste or even from the air and convert it into valuable products under benign conditions.
For over 150 years, scientists have been grappling with the unresolved problem of electrochemically converting carbon dioxide. Previous studies have mainly concentrated on developing catalyst materials and understanding the energy-intensive CO2 conversion reaction in water-based electrolytes. However, water-based systems have limitations in terms of CO2 capacity, and they often produce undesirable side reactions, such as hydrogen gas emissions.
In a recent study published in the European journal Angewandte Chemie, a team of researchers from Case Western Reserve University demonstrated a breakthrough in the electrochemical conversion of CO2 using ionic liquids. Ionic liquids are salts that melt below 100°C, and the ones developed by this specific research group are liquid at room temperature.
The key advantage of these ionic liquids lies in their high capacity for CO2 capture and their ability to maintain electrochemical stability. By leveraging these unique properties, the team successfully achieved the desired electrochemical process. This breakthrough has the potential to revolutionize CO2 conversion by offering a more efficient and environmentally friendly alternative to traditional electrolysis.
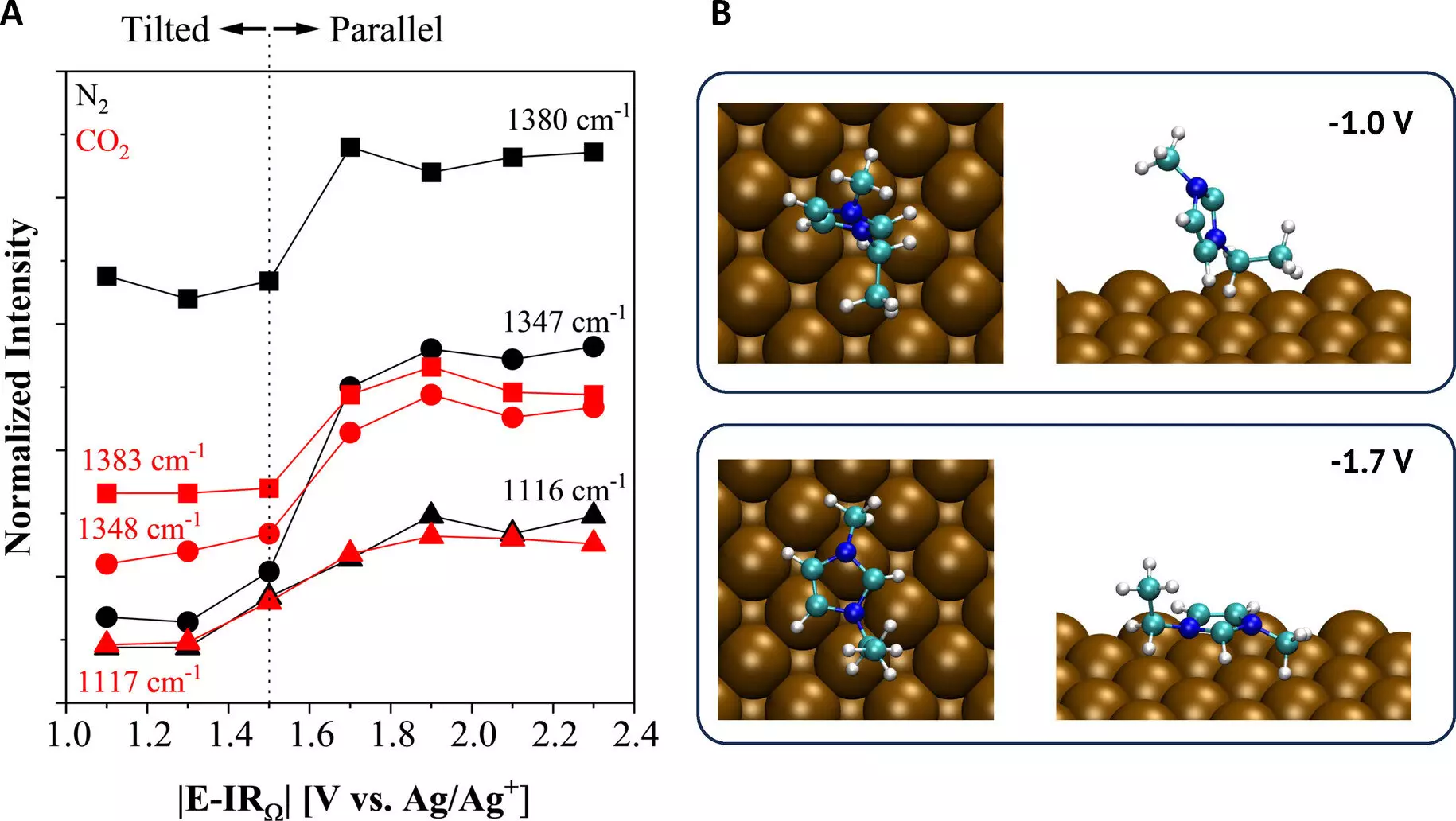
The research team, led by doctoral student Oguz Kagan Coskun, employed a combination of spectroscopic and electroanalytical techniques to gain insights into the fundamental mechanisms behind the activation of CO2 reduction at the copper electrode surface. This comprehensive understanding of the reaction is crucial for developing improved electrolyte designs and achieving better control over the resulting chemicals.
Additionally, the team’s study shed light on the various factors that influence the properties of the reaction environment and the effective use of CO2. This valuable information contributes to a deeper understanding of the reaction process and paves the way for the exploration of unconventional electrolytes.
The potential applications of this groundbreaking research are vast. With the ability to capture and convert CO2 into valuable products, electrochemical conversion using ionic liquids could revolutionize various industries. From the production of commodity chemicals to the development of renewable fuels, the possibilities are endless.
In addition to reducing greenhouse gas emissions, this innovative approach offers a more energy-efficient and cost-effective solution. By requiring less energy to drive the reaction and eliminating the unwanted side products associated with traditional electrolysis, this technology could significantly contribute to a more sustainable future.
The research team at Case Western Reserve University plans to delve deeper into the individual reaction steps and use the knowledge gained to inform the design of subsequent electrolytes. This ongoing research aims to refine and optimize the electrochemical approach to CO2 recycling, further enhancing the control and efficiency of the chemical production process.
As the world continues to grapple with the challenges posed by climate change, advancements in CO2 conversion technologies become increasingly crucial. The efforts of researchers at Case Western Reserve University are a testament to the immense potential of electrochemical conversion using ionic liquids. By harnessing renewable energy sources and developing sustainable processes, we can pave the way towards a greener and more sustainable future.


Leave a Reply