Fluorinated gases, known for their hazardous nature and ozone-depleting effects, have long posed a challenge in terms of safe handling and storage. However, a recent study conducted by chemists from Cornell University, the Korea Institute of Science and Technology, and Southern Methodist University has discovered a groundbreaking solution. By utilizing metal organic frameworks (MOFs), the research team has managed to develop a safer and cleaner method for handling these harmful reagents.
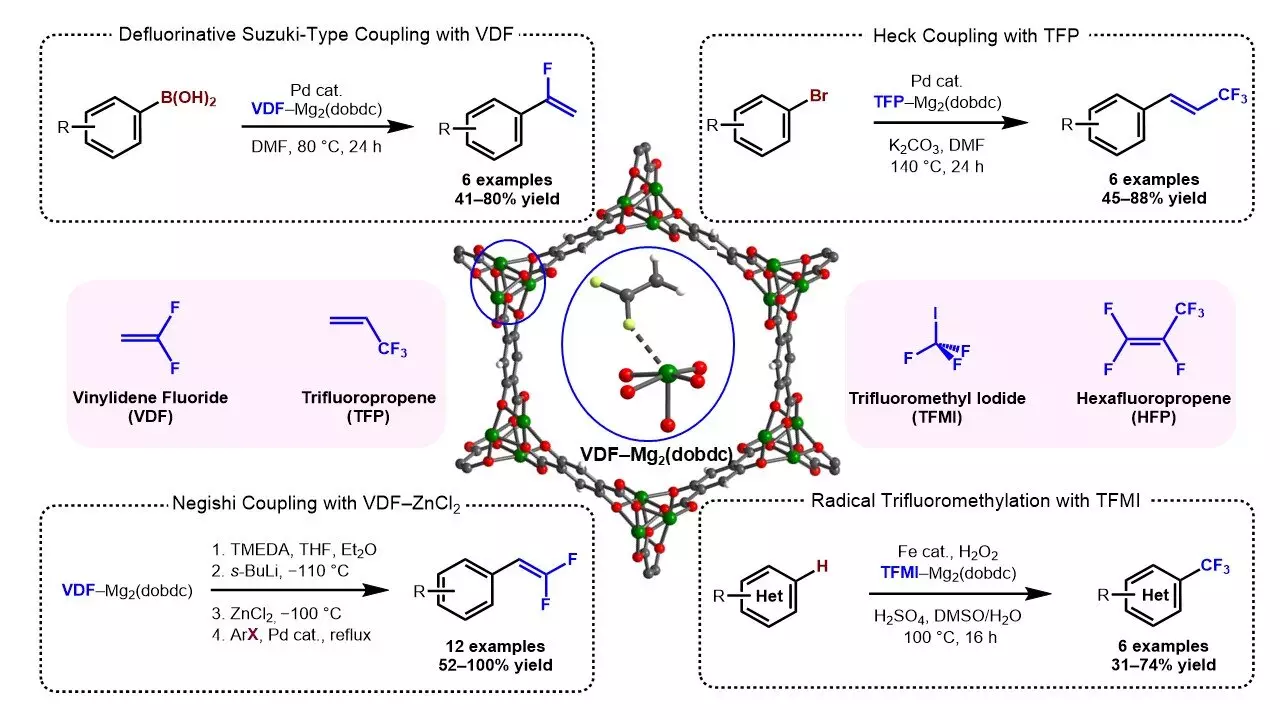
The objective of the research team was to find a suitable material that could effectively hold fluorinated gases without leaking. They sought a substance that was both porous and had a high gas sorption capacity, ensuring the gases remained contained. Moreover, the material needed to remain stable over a reasonable period of time. Thus, the team undertook extensive testing on 12 specific MOFs, specifically examining the uptake of vinylidene fluoride – a prevalent fluorinated gas.
Among the various MOFs analyzed, one named Mg2 exhibited the most promising characteristics. Not only did it possess high gas sorption capacity, but it also formed strong interactions with multiple fluorides. Furthermore, the Mg2 MOF showcased remarkable durability and stability. To test its viability, the researchers left Mg2 on a benchtop for an entire week, finding it remained intact and functional. Importantly, the gases contained within the MOF could be readily released by submersion in a solvent.
After creating gas-MOF reagents, the team made an intriguing observation – the gases could be contained within the MOFs for up to a week without any leakage. However, they wanted to explore the potential for even longer containment. By embedding the MOFs in wax, they discovered that the gases could be safely held for up to two months. Remarkably, accessing the gases required nothing more than applying sonication.
The discovery of using metal organic frameworks as a means to safely handle fluorinated gases marks a significant milestone in the field of chemistry. With the ability to securely contain these hazardous reagents, the utilization of MOFs opens up new possibilities in the development of cleaner and safer industrial processes. The breakthrough not only addresses the challenges of handling flammable and toxic gases but also minimizes their ozone-depleting impact when released into the atmosphere.
The collaborative efforts of chemists from Cornell University, the Korea Institute of Science and Technology, and Southern Methodist University have yielded a groundbreaking solution for the safe handling of fluorinated gases. The discovery of the highly efficient and stable Mg2 MOF demonstrates the potential of metal organic frameworks in storing and containing hazardous reagents. With further research and development, this breakthrough could revolutionize industrial processes and contribute to a cleaner and safer environment.


Leave a Reply