Breast and gynecological cancers continue to be a significant threat to the health and well-being of women worldwide. In recent research published in the Journal of Medicinal Chemistry, MYT1 has emerged as a promising therapeutic target for these devastating diseases. Supported by Insilico Medicine’s AI-driven generative biology and chemistry engine, this study presents a significant breakthrough in the field of cancer research.
The identification of potential targets for new therapeutics is vital in the fight against cancer. With this goal in mind, a research team utilized Insilico’s proprietary AI-driven target identification platform, PandaOmics, to analyze data on multiple forms of gynecological cancers, including ovarian, endometrial, cervical, and breast cancer. Of particular interest was triple-negative breast cancer, known for its aggressive behavior and limited treatment options.
The research findings consistently ranked MYT1 at the forefront in terms of relevance across all diseases. MYT1 is a member of the Wee1-kinase family, exhibiting low expression in normal tissues but high expression in most cancer types. This unique characteristic led researchers to believe that MYT1 inhibition could serve as a promising synthetic lethality therapeutic strategy, especially in cancers with genome instability.
Despite the potential of MYT1 inhibition, its high similarity to Wee1 poses a significant challenge in designing selective MYT1 inhibitors. However, Insilico Medicine addressed this gap by leveraging Chemistry42, its AI-driven small molecule generation platform. Through the use of structure-based drug design (SBDD) strategies and stringent filters for similarity and selectivity, Insilico successfully designed a collection of compounds specifically targeting MYT1.
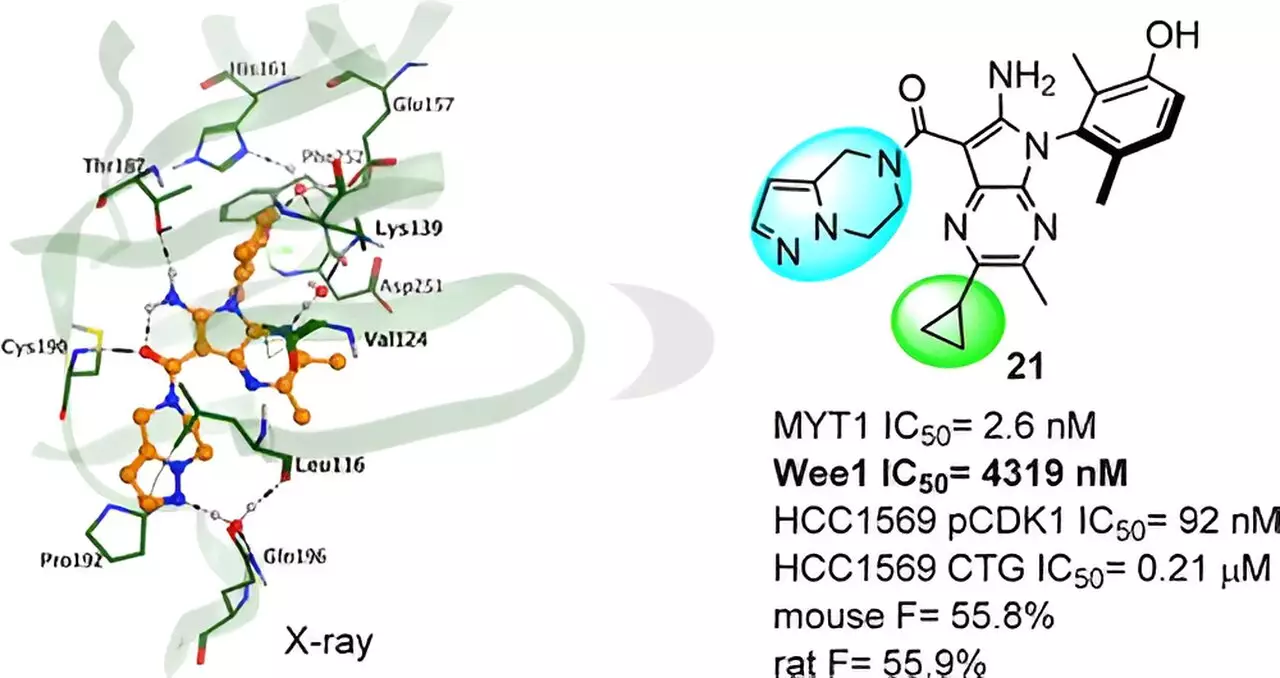
Among the novel compounds generated, a series emerged as hit compounds through extensive analysis. Insilico conducted an X-ray crystal structure analysis of the complex, revealing the impact of subtle chemical structure modifications on the activity of the compounds. This newfound knowledge guided further molecular optimization, ultimately leading to the discovery of the lead compound, Compound 21.
Compound 21 demonstrates good MYT1 activity and remarkable selectivity over Wee1, reducing the potential risk of off-target effects. Additionally, it exhibits favorable results in preclinical studies, displaying potent in vivo antitumor efficacy and a promising profile in absorption, distribution, metabolism, excretion, and pharmacokinetics (ADME and PK/PD). These findings pave the way for future development and clinical testing of Compound 21 as a selective MYT1 inhibitor.
Dr. Yazhou Wang, the medicinal chemistry leader of the MYT1 program from Insilico Medicine and the first author of the published paper, commends this innovative approach. He believes that this program not only provides an effective method for target identification but also holds the potential for the development of a promising selective MYT1 inhibitor.
The identification of MYT1 as a promising therapeutic target for breast and gynecological cancer marks a significant breakthrough in the field of cancer research. Through the utilization of AI-driven platforms and rigorous analysis, Insilico Medicine has successfully designed a series of novel compounds, culminating in the discovery of Compound 21. With its strong MYT1 activity and selectivity, Compound 21 holds great promise for the future of cancer treatment. As further research and development unfold, these findings pave the way for more effective therapies and improved outcomes for patients battling breast and gynecological cancers.

Leave a Reply