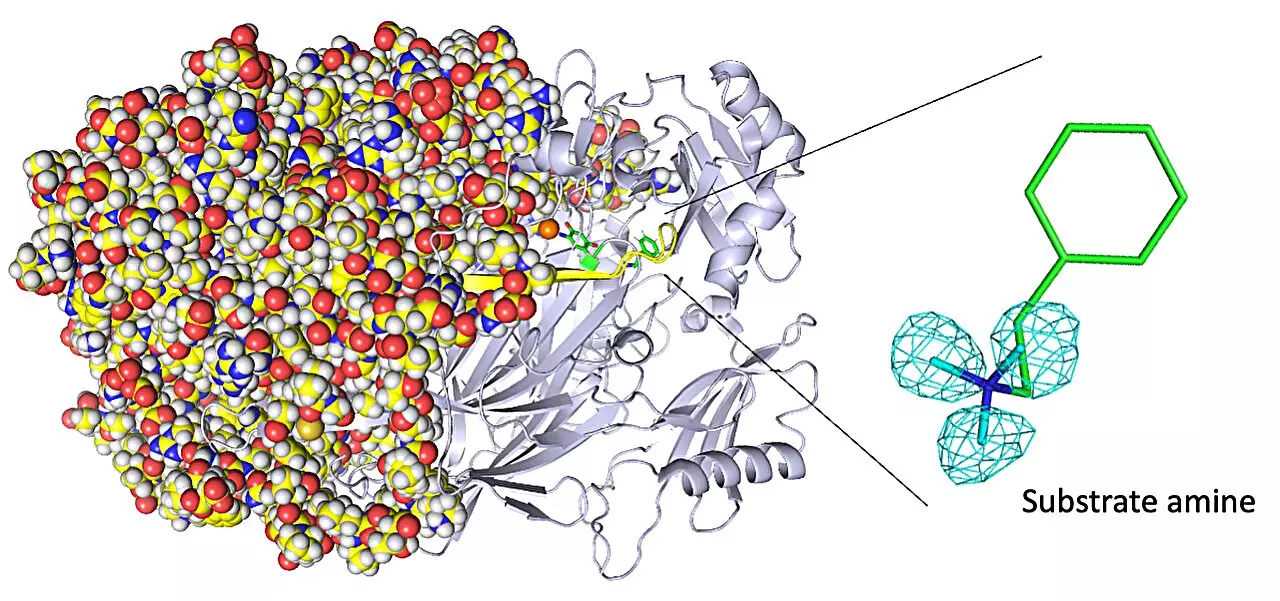
Copper amine oxidases, a class of enzymes, play a vital role in the healing process of wounds and detoxification of harmful substances in the human body. Understanding the atomic structure of these enzymes is crucial for enhancing their functionality. However, conventional imaging techniques struggle to accurately depict the positions of hydrogen atoms within these enzymes. In a groundbreaking study titled “Neutron crystallography of a semiquinone radical intermediate of copper amine oxidase reveals a substrate-assisted conformational change of the peptidyl quinone cofactor,” researchers from Osaka Medical and Pharmaceutical University and Osaka University have utilized neutron crystallography to observe the atom-by-atom structure of a copper amine oxidase enzyme, providing unprecedented insights into its biochemistry.
While copper amine oxidase enzymes exhibit unique biochemistry, such as quantum tunneling, their atomic structure remains elusive due to the inherent limitations of conventional imaging methods. X-ray crystallography, the most commonly used technique for determining enzyme structures, relies on electron diffraction and fails to capture hydrogen atoms effectively. To overcome this challenge, the research team turned to neutron crystallography, a technique that analyzes diffraction from atomic nuclei. By using this alternative imaging method, the researchers were able to obtain detailed structural information, including the elusive positions of hydrogen atoms.
The study yielded significant insights into the biochemistry of copper amine oxidase enzymes. It allowed researchers to visualize the protonation/deprotonation state of key sites within the enzyme, shedding light on their role in stabilizing radical species. These radical species are highly reactive atoms with unpaired electrons. Additionally, the researchers characterized the movement of the enzyme’s topaquinone cofactor, which facilitates single-electron transfer. They observed sliding, upward tilting, and half-rotation motions that contribute to the enzyme’s functionality. Moreover, the team made a groundbreaking discovery, uncovering the binding of a second molecule of high-affinity amine substrate during enzymatic reaction, a previously unknown event within the enzyme’s active site.
By revealing the precise positions of hydrogen atoms, this study helps explain the exceptional efficiency of copper amine oxidase enzymes at physiological temperatures and pressures. These newfound structural details pave the way for potential applications in various fields. The insights gained from this research could assist in the design of artificial enzymes, which can be utilized in industries such as pharmaceuticals and chemical manufacturing. With a deeper understanding of the biochemistry of copper amine oxidase enzymes, scientists can strive towards engineering improved enzymes with enhanced reactivity and functionality.
The application of neutron crystallography to study copper amine oxidase enzymes has provided unprecedented insights into their atomic structure. By overcoming the limitations of traditional imaging techniques, this study has revealed the positions of hydrogen atoms, shedding light on the biochemistry and functionality of these enzymes. The findings hold immense potential for the development of artificial enzymes and can revolutionize industries that rely on enzymatic processes. With a deeper understanding of these enzymes, researchers can continue to unlock the mysteries of biochemical reactions and pave the way for innovative scientific advancements.


Leave a Reply