Over the years, researchers at the University of Illinois Urbana-Champaign have been at the forefront of groundbreaking studies in the field of solvation and ion binding. In their latest publication in JACS Au, these researchers have shed light on a new pathway for electrochemically controlling ion selectivity by manipulating solvation. By expanding on their previous work on electrochemical separations of ions, the team, led by Professor Xiao Su and recent Ph.D. graduate Raylin Chen from the Department of Chemical & Biomolecular Engineering, discovered that solvation plays a critical role in the binding of ions. Through a unique approach utilizing a copolymer system, they have paved the way for a more precise and efficient method of ion separations.
Understanding the mechanisms behind ion binding is paramount in the development of efficient water treatment and resource recovery technologies. The researchers found that solvation, the process of surrounding the solute with solvent molecules, is a key factor in the binding of ions. By controlling solvation, it becomes possible to selectively bind different ions.
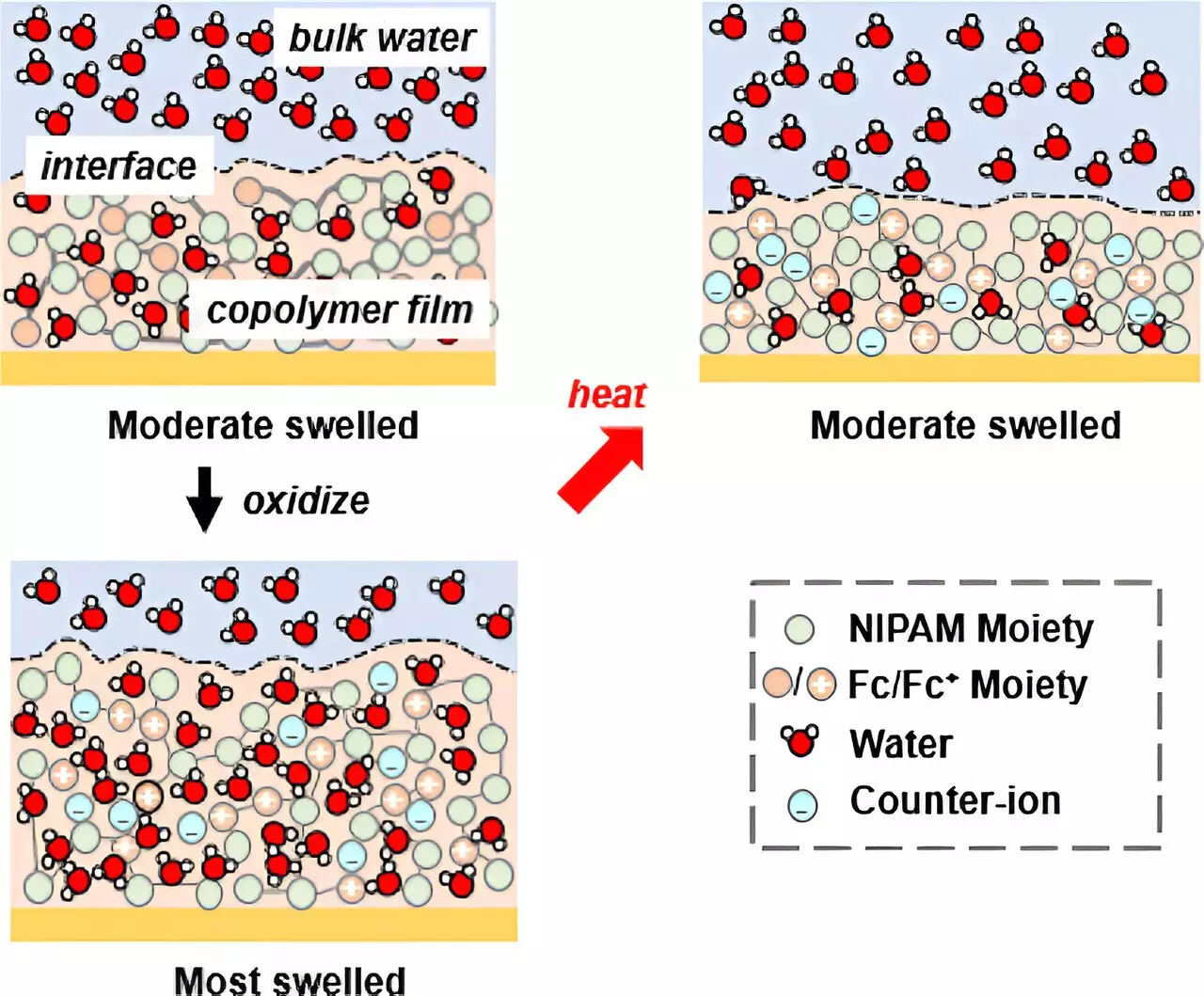
The team at the University of Illinois Urbana-Champaign devised a novel approach to control solvation and achieve ion selectivity. They introduced redox-active units to a copolymer system containing N-isopropyl acrylamide (NIPAM), a temperature-responsive material. The inclusion of redox-active units allowed dual pathways for controlling solvation. By adjusting the potential, the researchers were able to either force NIPAM to absorb water or release water through an electrochemical transition. This groundbreaking method opened up new possibilities for manipulating ion selectivity based on the degree of water uptake by the copolymer.
To validate their findings, the researchers utilized advanced techniques such as in situ ellipsometry and neutron reflectometry (NR). In situ ellipsometry, a method developed by the team, enabled them to observe the swelling and deswelling of gel films in response to the addition or release of water through electrochemistry. On the other hand, neutron reflectometry, a highly sensitive technique to water content, allowed the researchers to study solvation in depth. By analyzing the distribution of water across the film under different potentials, they were able to confirm the correlation between solvation control and ion selectivity.
The implications of this study go beyond the laboratory setting. The ability to control ion selectivity based on both temperature and electrochemical potential opens up possibilities for future sustainable applications. With the growing need for energy-efficient and selective technologies, the copolymer system developed by the team at the University of Illinois Urbana-Champaign provides a promising platform for water treatment and resource recovery. By harnessing renewable energy or waste heat, this material platform can be utilized in diverse scenarios, making it a potential game-changer in the field.
The study conducted by the researchers at the University of Illinois Urbana-Champaign represents a significant breakthrough in the understanding and control of ion selectivity through solvation. By manipulating solvation using a copolymer system, the researchers demonstrated the ability to selectively bind ions based on the degree of water uptake. The experimental validation using in situ ellipsometry and neutron reflectometry further confirmed the effectiveness of this approach. Looking ahead, this research lays the foundation for the development of more energy-efficient and selective technologies for water treatment and resource recovery. With a deeper understanding of the molecular mechanisms behind ion binding, the stage is set for advancements in the field of electrochemical separations.

Leave a Reply