Chemists from Oxford University and IBM Research Europe-Zürich have achieved a significant milestone in the field of chemistry. In their recent study published in the esteemed journal Nature, the researchers successfully synthesized a doubly anti-aromatic C16 carbon allotrope. This breakthrough opens up new avenues for exploration and experimentation in molecular chemistry, as well as potential advancements in the development of novel materials.
Previously, in 2019, another team of chemists managed to synthesize a new molecular form of carbon, C18, which exhibited a circular structure with alternating triple and single bonds. The success of this experiment hinted at the possibility of synthesizing a C16 carbon allotrope. However, previous attempts were thwarted by the inherent instability of the process.
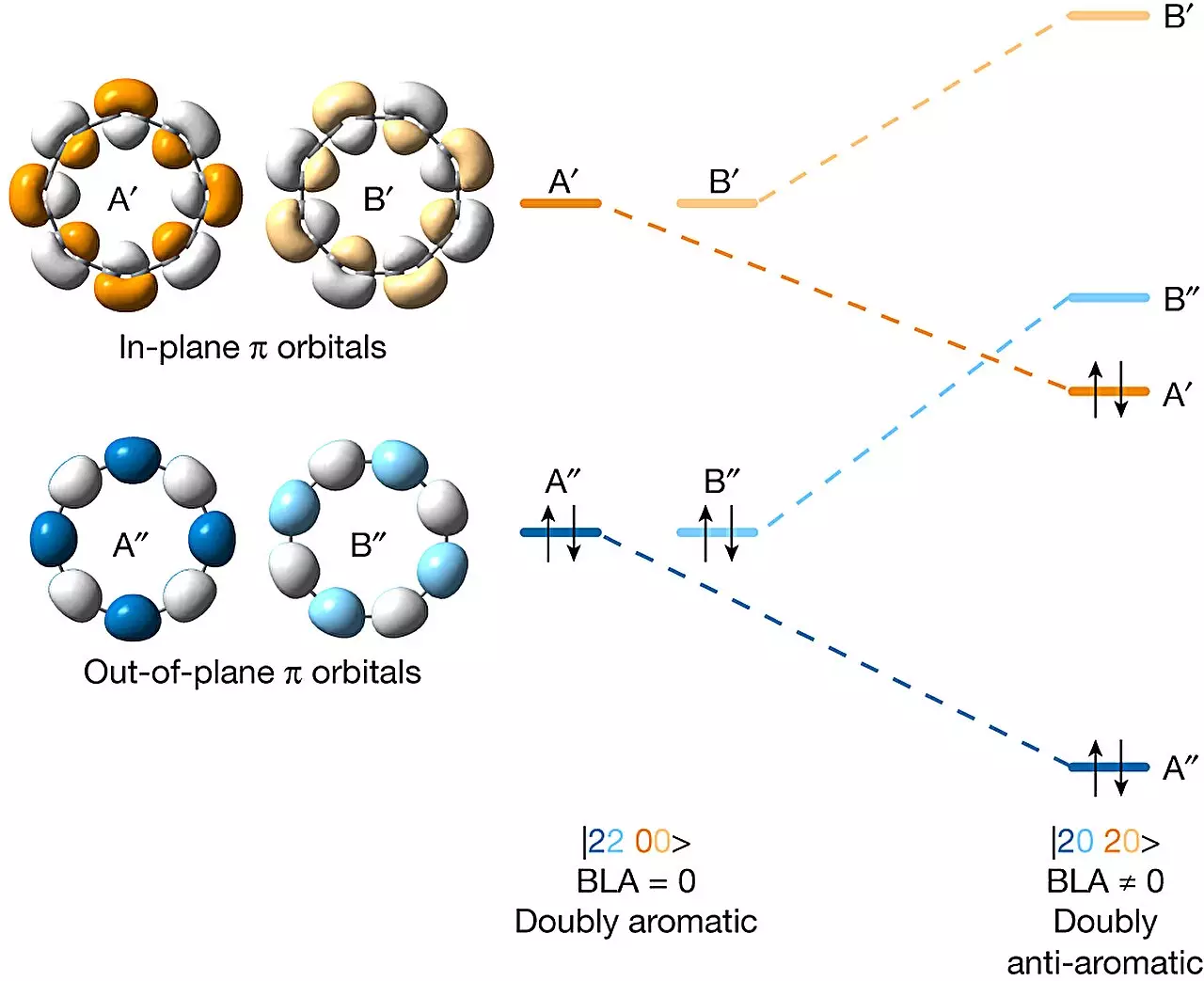
Undeterred by the challenges, the research team embarked on their mission to create a doubly anti-aromatic C16 carbon allotrope. To address the precursors’ instability, the researchers devised a novel approach. They employed several masking agents to enhance stability, successfully overcoming the high-energy levels associated with anti-aromatic structures.
The final synthesis process involved utilizing a macrocycle with two bromine and four carbon monoxide substituents. These components were layered onto a sodium chloride substrate. To trigger the formation of bonds between carbon atoms, the researchers employed a tunneling microscope to deliver picoamperes of charge to specific sites on the molecule. This method raised the voltage on the bromine to 1.3V and the carbon monoxide to 3V, enabling the desired bonds to form.
Following the successful synthesis, the team verified the creation of the doubly anti-aromatic C16 carbon allotrope. They utilized an atomic force microscope to focus on the ring structure, confirming its formation. Additionally, they employed a Kelvin probe force microscope and a scanning tunneling microscope to examine the molecular orbitals’ electron distribution. These observations demonstrated that the molecule exhibited the expected doubly anti-aromatic characteristics.
This groundbreaking achievement has the potential to revolutionize the field of chemistry by opening opportunities for further research and exploration of new theories. The doubly anti-aromatic C16 carbon allotrope could serve as a catalyst for the development of novel materials with extraordinary properties and applications. Researchers can now delve deeper into the realm of exotic carbon allotropes, shedding light on their unique structures and behaviors.
The successful synthesis of a doubly anti-aromatic C16 carbon allotrope by the collaborative team from Oxford University and IBM Research Europe-Zürich marks an unprecedented breakthrough in chemistry. This accomplishment not only expands our understanding of carbon allotropes but also paves the way for new frontiers in experimental theories and material development. With this achievement as a foundation, the future holds immense promise for uncovering the mysteries of molecular chemistry and unlocking the potential of carbon-based materials.


Leave a Reply