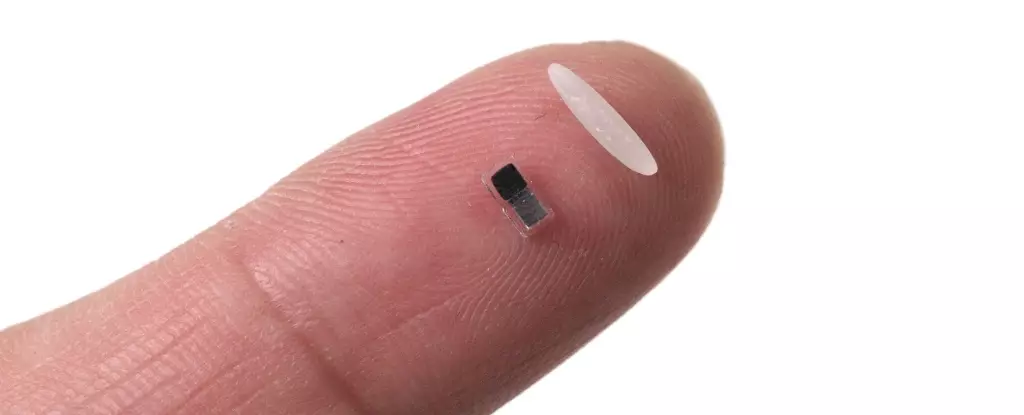
In a stunning advance that promises to reshape cardiac treatment across the globe, researchers have unveiled an incredibly tiny pacemaker, measuring smaller than a grain of rice. This innovative device, still in its nascent stages and not yet ready for human trials, represents a significant leap in the treatment of heart conditions, particularly for vulnerable populations such as infants with congenital heart defects. What sets this wireless pacemaker apart is not only its miniature size but also its groundbreaking ability to be injected and controlled through light, followed by its safe dissolution within the body. This approach could potentially eliminate the need for invasive surgeries that carry risks and complications.
Addressing Unmet Needs in Cardiac Treatment
Currently, the process for implanting temporary pacemakers involves surgical intervention where electrodes are sewn onto the heart muscles, requiring cumbersome connections to external devices that can lead to further health complications. A stark reminder of the risks involved was the 2012 death of Neil Armstrong, caused by internal bleeding after his temporary pacemaker’s removal. The urgency for safer alternatives has never been clearer, underscoring the new pacemaker’s timely introduction. Designed to provide immediate support following heart surgery, this breakthrough could drastically improve the recovery process for both children and adults.
How Does It Work?
At the core of this innovation lies a fascinating technology that employs a combination of light activation and biocompatible materials. Once injected, the pacemaker is paired with a soft, wearable patch on the patient’s chest. This patch plays a crucial role, monitoring the heart’s rhythm and employing light signals to communicate with the pacemaker, instructing it on how to regulate heartbeats effectively. This seamless integration eliminates the need for wires and cumbersome external devices, thereby enhancing patient comfort and safety. The pacemaker operates through a galvanic cell, utilizing the body’s own fluids to transform chemical energy into electrical stimuli, paving the way for a truly autonomous cardiac support system.
A Broad Horizon for Biomedical Applications
The implications of this technology extend well beyond cardiology. With researchers suggesting its potential applications in fields like nerve regeneration and wound healing, we stand on the precipice of a paradigm shift in medicine. As Bozhi Tian, a researcher from the University of Chicago, points out, this development serves as a substantial progress marker in bioelectronic medicine. The innovative device may inspire researchers to develop smart implants that can address various health challenges, creating an integrated system that enhances patient care and ultimately saves lives.
Looking Ahead: The Path to Human Trials
With successful tests conducted on various animals, including mice and dogs, the timeline for human trials is fast approaching. Senior study author John Rogers of Northwestern University has optimistically projected that testing could begin within two to three years, a timeline that emphasizes both urgency and hope. As a significant player in bringing this technology to life, Rogers has formed a startup focused on navigating the hurdles to human application. This initiative underscores the importance of collaboration between academic research and business endeavours to bridge the gap from laboratory to clinical settings.
Transforming the Medical Landscape
The announcement of this tiny pacemaker has sparked enthusiasm in the medical community, heralding a future where complexities in cardiac care can be managed with elegance and efficiency. As heart disease remains the world’s leading cause of death, this achievement not only highlights the potential for innovation in treatment paradigms but also reflects a vital step towards a healthcare system that prioritizes patient safety and improved outcomes. The continuous evolution of technology in medicine opens doors we never thought possible; this miniature pacemaker is just the beginning of a larger revolution in healthcare.

Leave a Reply