Water sources around the globe face increasing threats from pollution, particularly from heavy metals like cadmium and lead. These contaminants not only endanger aquatic ecosystems but also pose serious health risks to humans who consume affected water. Heavy metals can accumulate in the body, leading to chronic health issues such as neurological damage and other serious ailments. Traditional methods for removing these toxins, such as filtration systems, often fall short due to their extensive energy requirements and the rapid clogging of metal-capturing membranes. As a consequence, there is an urgent need for innovative solutions that can offer efficient, sustainable, and economically viable methods for water purification.
The Role of Sugar-Derived Polymers
Recent advancements in the field of water treatment have increasingly focused on natural and biodegradable materials, particularly those derived from plant sources. Researchers have discovered that polysaccharides—complex carbohydrates with repetitive sugar units—exhibit a capacity for binding heavy metals. These natural polymers can create physical barriers within plants, which predominantly contain macromolecules that effectively trap harmful metal ions. Previous research has demonstrated their efficacy in targeting smaller pollutants, such as microplastics, by utilizing sticky extracts from sources like okra and aloe. However, these polysaccharides often require additional substances to maintain their effectiveness in water, as many tend to dissolve, decreasing their utility in practical applications.
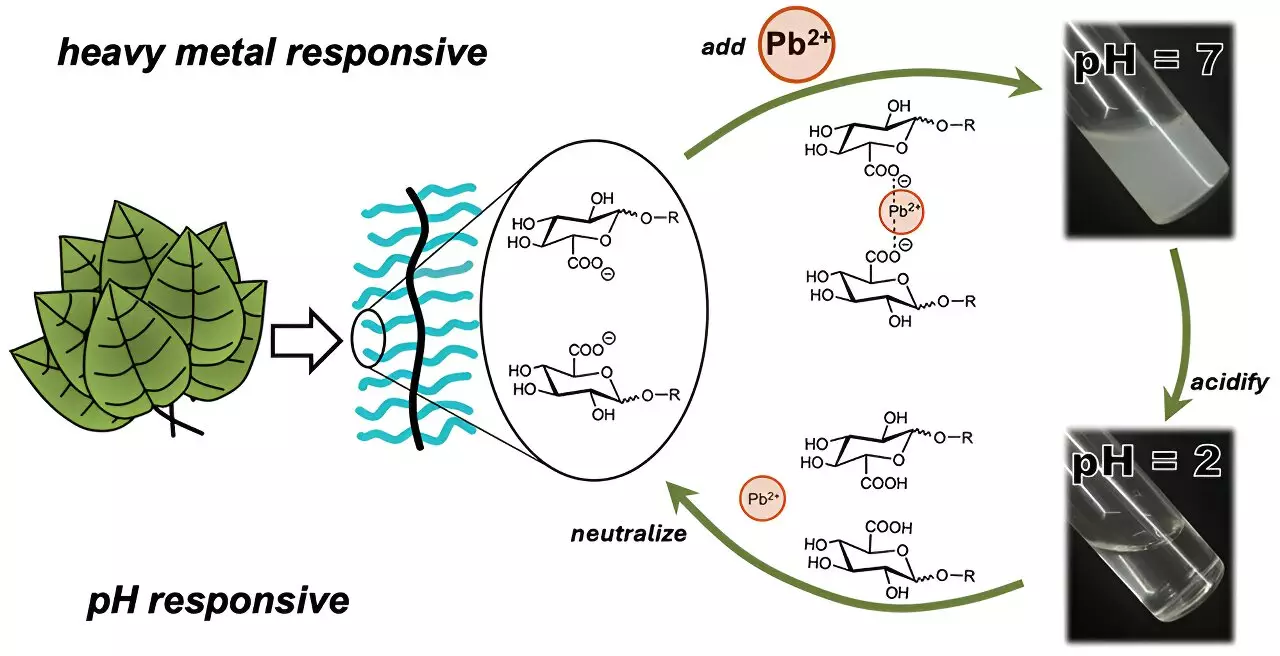
In a significant step towards addressing the limitations of traditional methods, researchers led by Cassandra Callmann at the University of Texas at Austin have engineered a new type of polymer that captures heavy metals while overcoming the solubility issues faced by earlier solutions. This novel polymer is characterized by an insoluble backbone with various water-soluble carbohydrate “charms” attached, akin to a charm bracelet. The most effective carbohydrate for binding heavy metals contained a carboxylic acid group, which significantly enhanced the polymer’s ability to attract and hold ionic cadmium.
In groundbreaking initial tests, the team demonstrated that the polymer could effectively trap cadmium from water samples within minutes, forming visible clumps that could be easily filtered out. Remarkably, they also showed that adjusting the acidity of the water could trigger the release of the trapped cadmium, allowing for both recycling and reusability of the polymer itself—an essential feature for sustainable water purification practices.
Building upon their successful laboratory results, the research team conducted a proof-of-concept test using water from the Colorado River that had been artificially contaminated with specific amounts of ionic cadmium and lead. The river water sample, inherently rich in other metal ions like calcium, sodium, and magnesium, created a challenging environment for any metal extraction method. Nonetheless, after a 24-hour exposure, the innovative polymer managed to capture an impressive 20% of the cadmium and 45% of the lead without significantly affecting the concentrations of other essential metal ions.
The results from this study not only establish the polymer’s effectiveness in complex water conditions but also hint at its potential for use in diverse environments where heavy metal contamination is prevalent.
The implications of this research are profound. The novel polymer represents a crucial advancement towards developing efficient, selective, and recyclable materials for water purification systems. As society continues to grapple with the repercussions of industrialization and urban pollution, solutions like this will be essential in safeguarding public health and restoring ecological balance.
Callmann’s team’s work encompasses both innovative chemistry and a deep understanding of plant biology to pave the way for cleaner waterways. By focusing on sustainability and efficiency, this research signifies a promising future for water purification technologies, adding to the growing arsenal of tools available to combat the pervasive issue of water contamination. As the technology advances, we may find far-reaching benefits for both human populations and aquatic ecosystems alike.

Leave a Reply