In the pursuit of sustainable energy solutions and the combat against climate change, scientists are increasingly exploring the potential of artificial photosynthesis. This innovative technology mimics the natural process by which plants convert sunlight, water, and carbon dioxide into glucose and oxygen. A recent advancement from the University of Michigan has demonstrated a promising system that not only recycles CO2 but also converts it into valuable hydrocarbons, specifically ethylene. This breakthrough offers an exciting glimpse into how we might address the dual challenges of plastic production and carbon emissions through a more eco-friendly method.
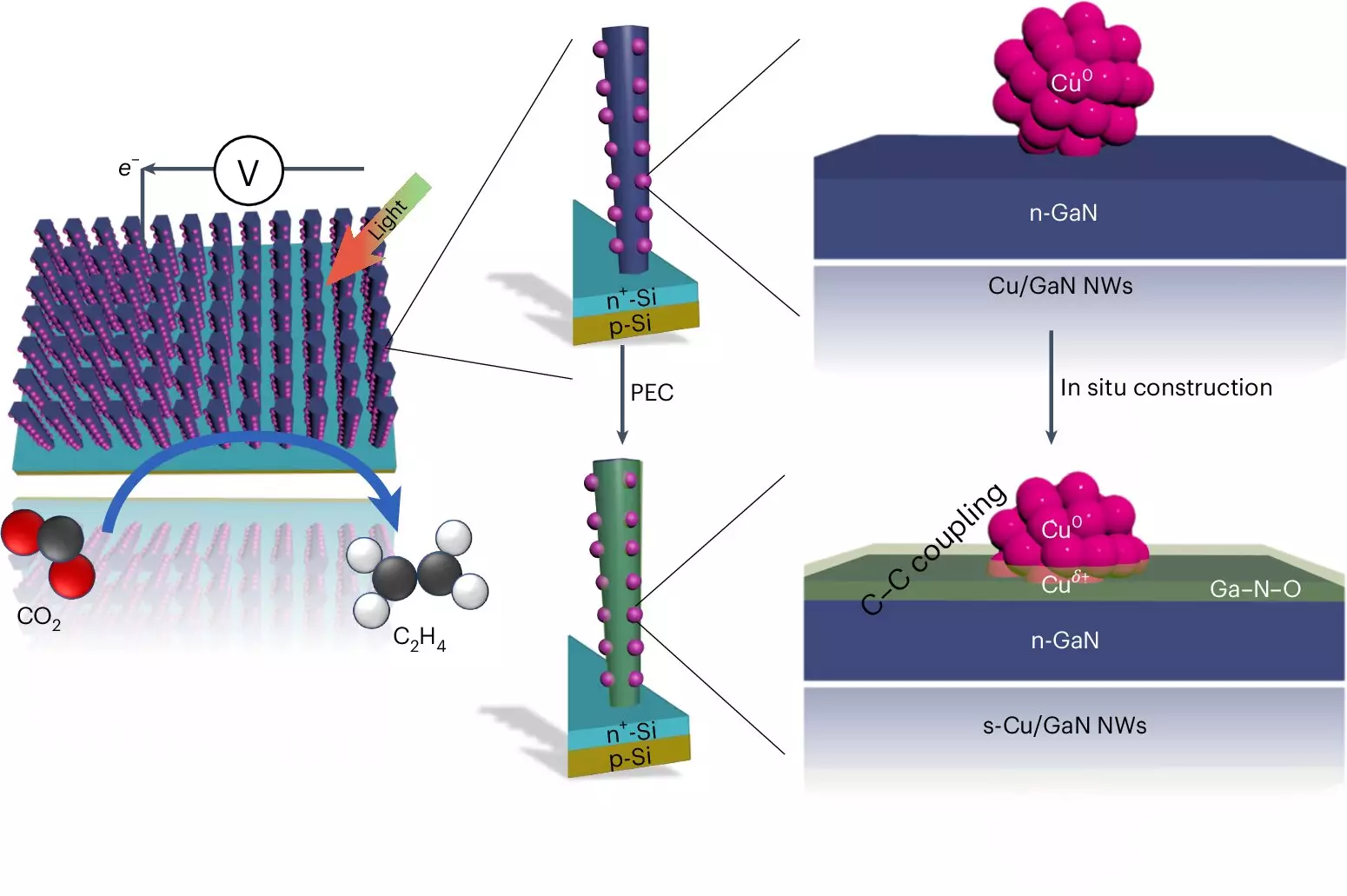
The University of Michigan’s system operates on a remarkable process that binds two carbon atoms together to produce ethylene—a compound that plays a critical role in the plastics industry. Traditionally, ethylene has been produced from fossil fuels under extreme conditions that generate significant CO2 emissions. The artificial photosynthesis system developed by the research team outpaces previous attempts by achieving a performance rate five to six times higher than the prevailing technologies. The innovation lies in a highly efficient setup involving gallium nitride nanowires and a copper catalyst, which work synergistically to drive the reaction.
The nanowires, measuring merely 50 nanometers in diameter, act as a platform for capturing light, which is essential for splitting water molecules into hydrogen and oxygen. This splitting takes place at the surface of the nanowires when exposed to simulated sunlight, creating the necessary conditions for the production of hydrocarbons. The captured hydrogen then interacts with carbon monoxide, derived from CO2, ultimately forming ethylene.
One of the standout features of the new system is its longevity. While many existing catalysts degrade rapidly, limiting their practical applications, the Michigan team’s device has shown impressive endurance—operating for over 116 hours without significant loss of performance. Furthermore, it demonstrates a remarkable stability over extended durations, having reportedly functioned effectively in experiments for up to 3,000 hours. This extended operational capability is facilitated by the unique interaction between the gallium nitride and the water-splitting reaction, which fosters a self-healing process, bolstering the longevity of the system.
This remarkable endurance directly translates to economic viability, making the process not only environmentally sustainable but also potentially cost-effective. The ability to run for extended periods without degradation opens the door to industrial applications where consistency and reliability are crucial.
The long-term vision of this research goes beyond ethylene production. The team at the University of Michigan aims to further develop the technology to facilitate the synthesis of longer-chain hydrocarbons and liquid fuels such as propanol—substances that could greatly enhance energy sustainability in transportation. Transitioning from fossil fuels to renewable energy sources such as liquid fuels would dramatically reduce the carbon footprint of transportation technologies that are currently reliant on oil and gas.
The ability to harvest CO2 emissions for creating valuable products not only provides an innovative solution to one of the world’s pressing environmental issues but also represents an economically viable avenue for the plastics industry and beyond.
As the world grapples with the urgent need for sustainable energy solutions, advancements like those from the University of Michigan serve as beacons of hope. The implications of this research extend far beyond basic science; they have the potential to reshape entire industries. Moving forward, further research will focus on increasing the efficiency of the process even more and expanding the range of hydrocarbons that can be synthesized through this method.
Ultimately, success in this domain hinges on a collaborative effort between scientific research and industrial application—efforts that can bring about the transition to a carbon-neutral future where sustainability and economic viability coexist. As innovations in artificial photosynthesis continue to emerge, the dream of turning CO2 into a resource rather than a waste product increasingly appears within reach. The path forward is indeed filled with promise, paving the way for a cleaner, more sustainable world.


Leave a Reply