A ground-breaking development in explosives detection technology has been achieved by a team of scientists. They have devised a method to detect trace amounts of hard-to-detect explosives from distances of over eight feet, eliminating the need for close contact or physical contact with suspicious materials. This innovative method, outlined in the journal Talanta, has the capability to identify explosives like nitroglycerin and RDX through the air at exceedingly low concentrations within mere seconds.
The unique aspect of this technology lies in its exceptional sensitivity. The equipment utilized by the scientists is capable of recognizing minuscule amounts of explosives, as minute as 10 parts per quadrillion. To put this into perspective, this level of sensitivity is likened to being able to distinguish a single pine needle from an entire forest of pine trees in the state of Washington. Furthermore, it is equivalent to identifying a lone coin from a towering stack of pennies that surpasses the height of Mount Everest by 17 million times.
The recent breakthrough by researchers at the Department of Energy’s Pacific Northwest National Laboratory represents a significant advancement in the field of explosives detection. Over the years, the team has dedicated extensive efforts to detecting vapors emitted by various materials, particularly explosives. Previously, their methods enabled detection from a mere half-inch distance. However, the latest technology pushes the boundaries by recognizing the same substances from distances ranging from two to eight feet, depending on the material.
This cutting-edge technology has already been licensed to BaySpec Inc., a prominent instruments manufacturing company based in Silicon Valley. Specializing in spectral sensing for diverse applications including semiconductors, pharmaceuticals, defense, and security, BaySpec Inc. plans to develop a commercial product based on this technology for explosives and narcotics detection by the year 2025.
The team led by chemist Robert Ewing at PNNL has made significant strides in detecting materials with low vapor pressure through a technique known as standoff detection. This method specifically targets materials that do not easily evaporate, releasing minimal molecules into the air, thereby posing challenges in capture and analysis. In contrast, substances with higher vapor pressure such as gasoline readily become airborne, making them simpler to detect.
Enhanced Detection Capabilities
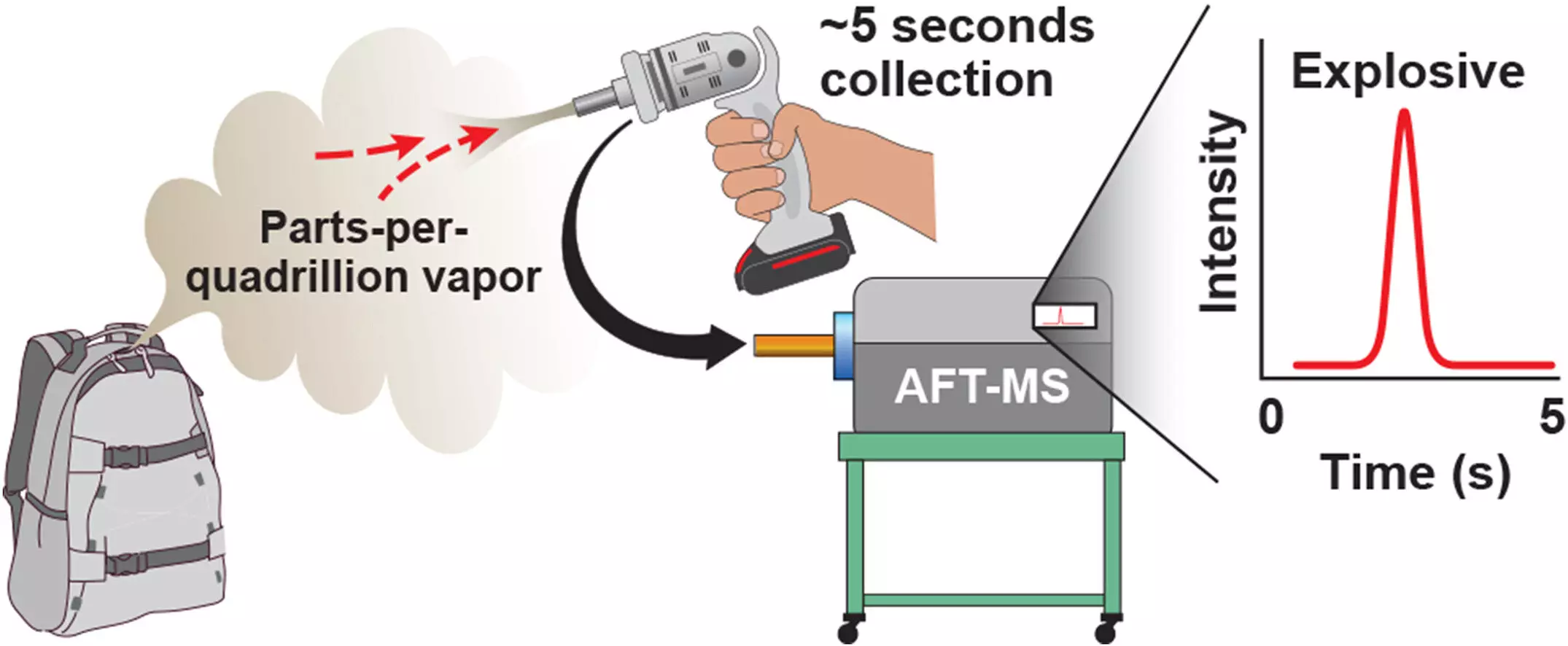
One key element contributing to the improved detection distance is a handheld air sampler devised at the University of Washington. This powerful sampler can draw in around 300 liters of air per minute, facilitating the collection of sufficient air in just 5 to 10 seconds for the detection of materials characterized by low vapor pressure. The collected air passes through a filter to capture the vapors, which are then directed to an atmospheric flow tube and subsequently identified by a mass spectrometer.
A critical component in the sensitive detection process is the atmospheric flow tube, a roughly two-foot-long device where molecules are ionized before being transmitted to the mass spectrometer. The extended distance of the tube allows for increased time for the target molecules to be ionized, significantly amplifying the sensitivity of detection. With the aid of this atmospheric flow tube, the team can pinpoint explosives at a level lower than 10 parts per quadrillion.
The team envisions that this groundbreaking technology, with its standoff detection approach, can be instrumental in protecting individuals from explosive threats. This remarkable achievement builds upon the team’s past successes, including recognition with an R&D 100 Market Disruptor Award. Members of the team recently participated in the Trace Explosives & Drug Detection Workshop to share insights and collaborate on related initiatives. This work forms a part of a broader program at PNNL focusing on explosives detection, showcasing the commitment to advancing science and technology for global security.

Leave a Reply